Basic Course of Thermo-Fluid Analysis 06: Chapter 3 Basics of Flow - 3.2.1 Compressible/incompressible fluids|List

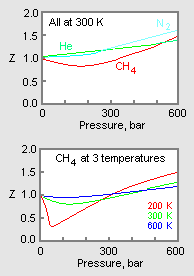
What is the compressibility factor? What is its value for an ideal gas? How does it help to understand the extent of deviation of a gas from ideal behavior?

The Behavior of Gases Chapter 14. Chapter 14: Terms to Know Compressibility Boyle's law Charles's law Gay-Lussac's law Combined gas law Ideal gas constant. - ppt download

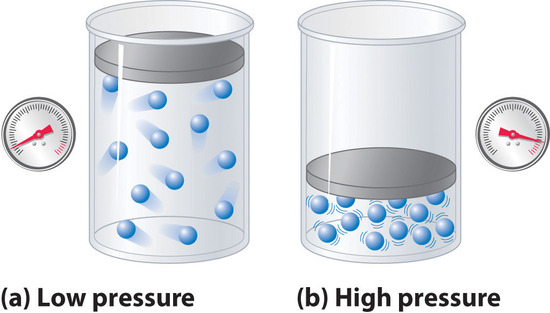
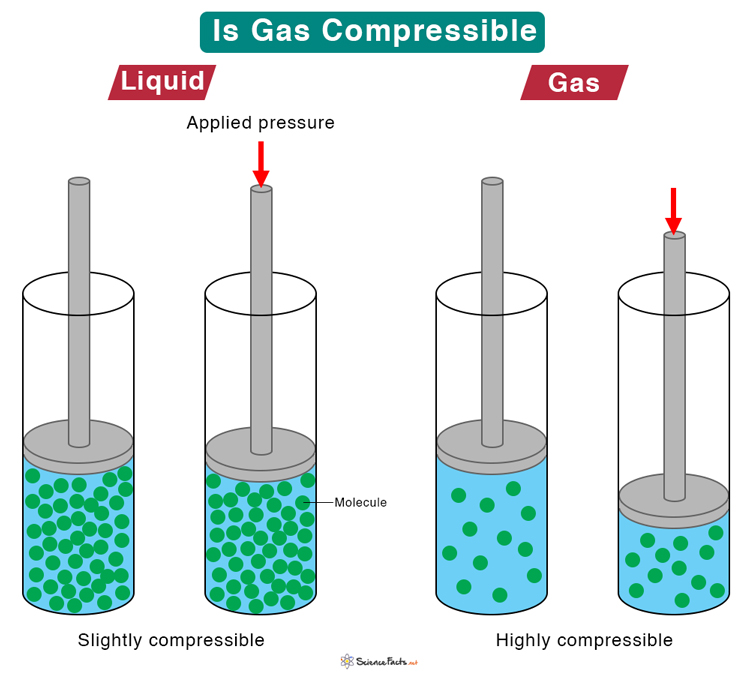
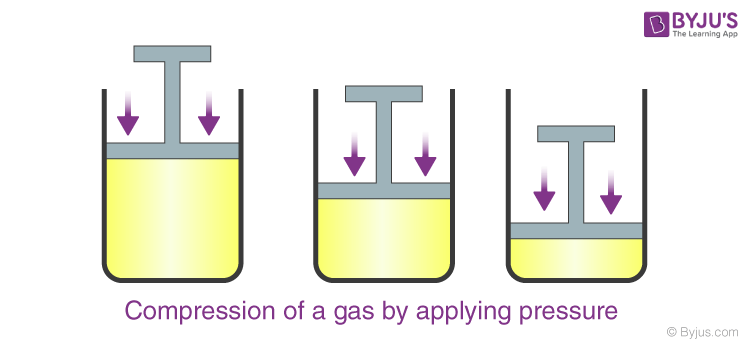
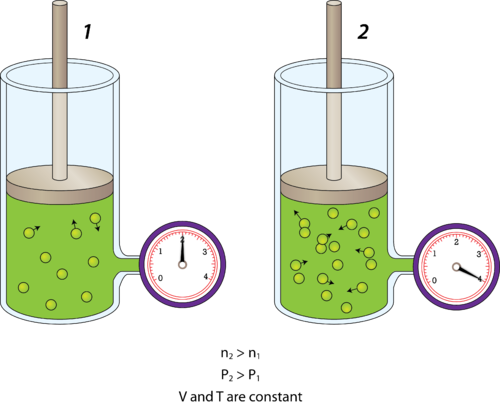
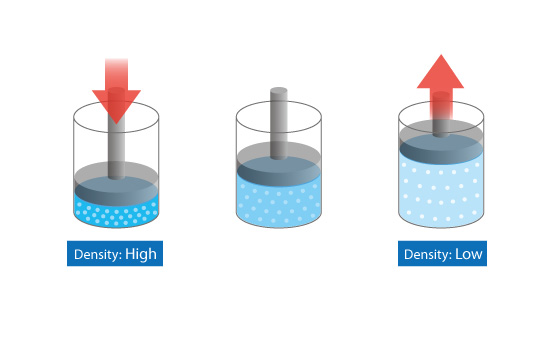
Jennie L. Borders. Section 14.1 – Properties of Gases Compressibility is a measure of how much the volume of matter decreases under pressure. Gases are. - ppt download

Aim: What are the properties of Gases?. Compressibility Compressibility is measure of how much volume decreases under increased pressure. Gases are easily. - ppt download

Design an activity to show that gases are compressible as compared to solids and liquids - Science - Matter in Our Surroundings - 9178247 | Meritnation.com

Properties of Gases | Mobility, Compressibility, Pressure, Density | in Urdu Hindi Lecture - YouTube


![Difference in Characteristics of States of Matter [in Table Format] Difference in Characteristics of States of Matter [in Table Format]](https://d1avenlh0i1xmr.cloudfront.net/712713a1-9772-428a-9ecd-85f384790b2e/arrangement-of-particles-in-solids,-liquids-and-gases-teachoo-01.jpg)