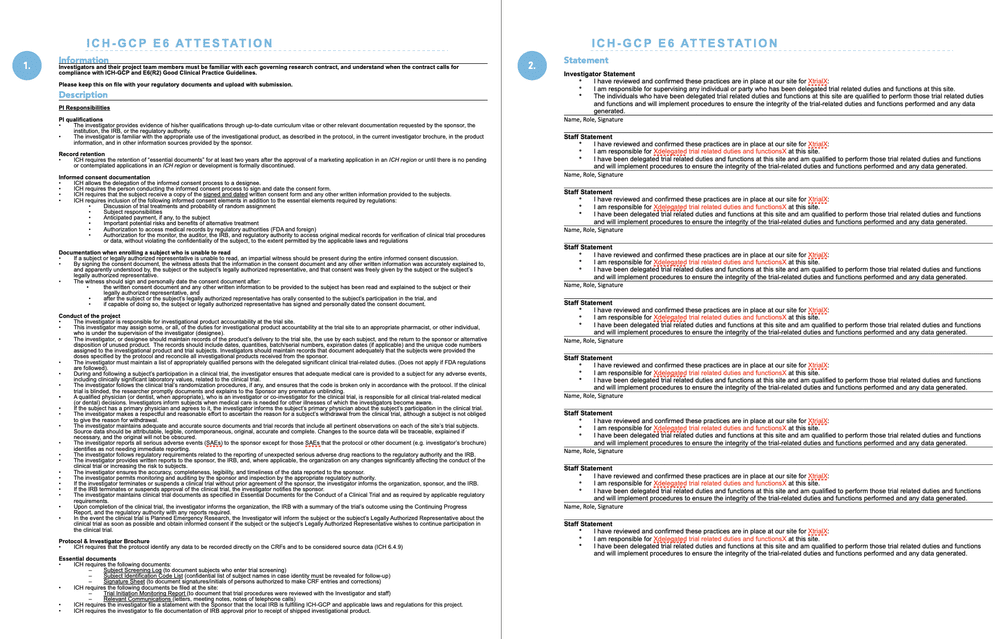
Safeguarding public health Lynne Byers An Introduction to MHRA and GCP Inspections – Lynne Byers Medicines and Healthcare products Regulatory Agency. - ppt download
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials
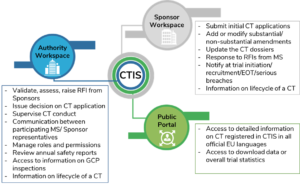
Adapting to the Evolving European Clinical Trial Regulatory Scenario: An Overview of the Current State of the European Clinical Trials Regulation and Clinical Trials Information System - ACRP

EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation: Molecular Therapy - Methods & Clinical Development

IMPD review process To ensure the implementation of GCP in the MS of EU... | Download Scientific Diagram
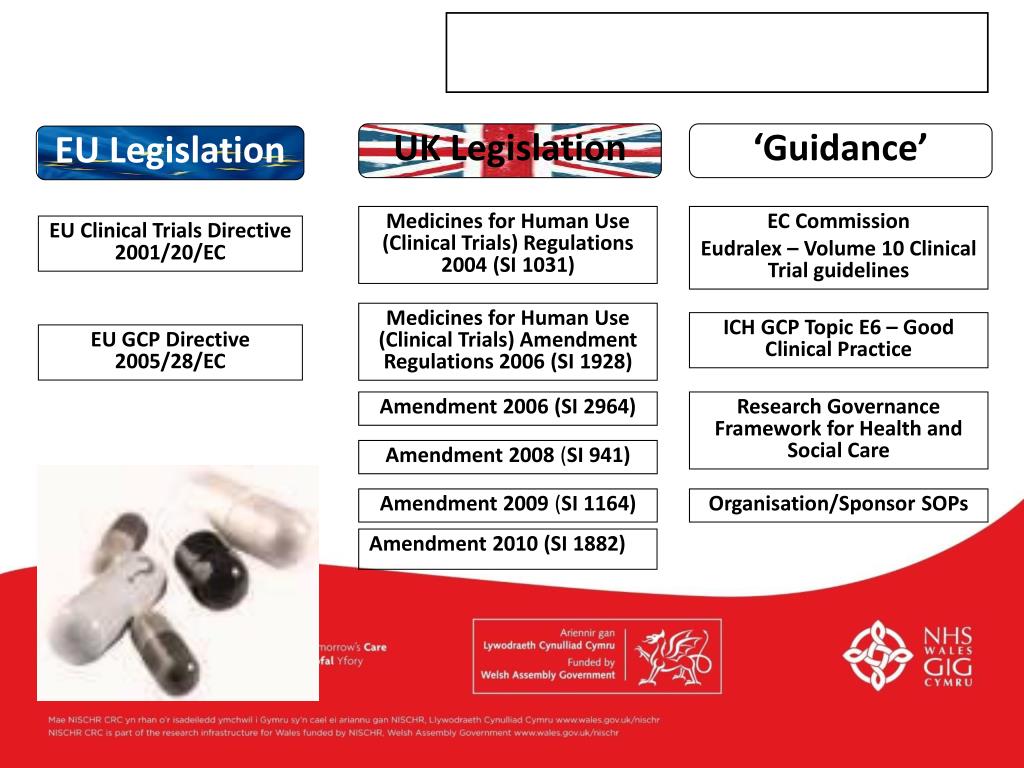
PPT - Good Clinical Practice in Research Clinical Trial Regulations PowerPoint Presentation - ID:776636

PDF) Scrip Regulatory Affairs The EU Clinical Trials Regulation: one step forward, two steps back | John Lisman - Academia.edu

GCP and Quality in “Regulation (EU) 536/2014 on clinical trials on medicinal products for human use and repealing Directive 2001/20/EU” - ScienceDirect

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library