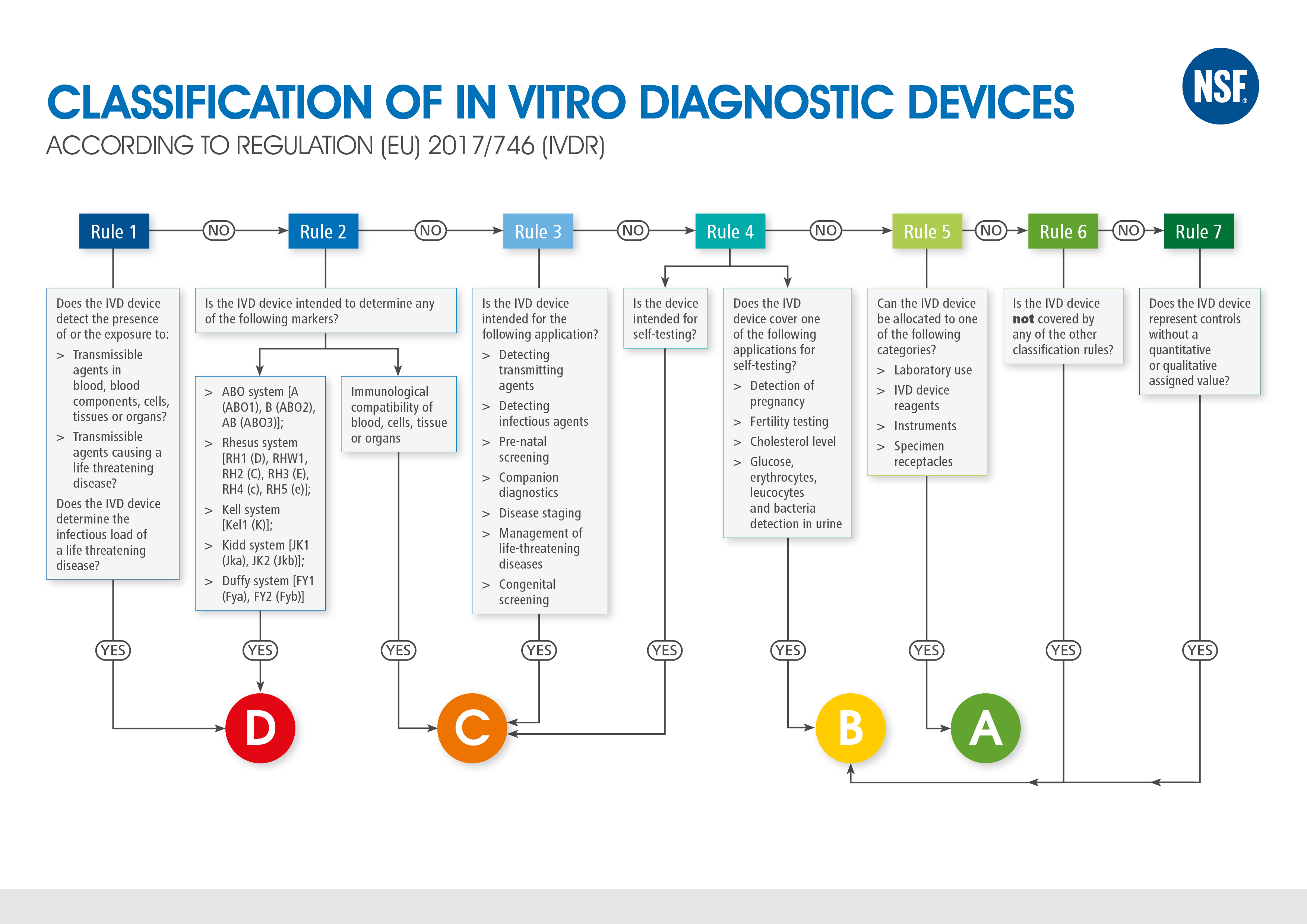
In Vitro Diagnostic Regulation (IVDR)Consulting Services - MakroCareRegulatory, Clinical Consulting Services to Biopharma & Medical Device Companies | MakroCare

Implementation of the new EU IVD regulation – urgent initiatives are needed to avert impending crisis

IVD Directive to IVD Regulation (EU 2017/746) Transition – 8 Months Remaining - Voisin Consulting Life Sciences






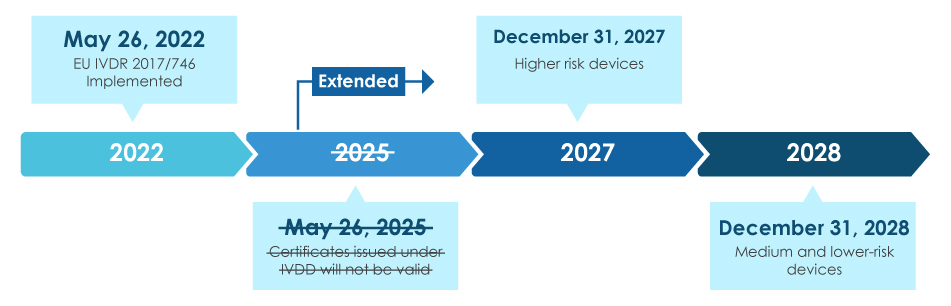



.jpg)


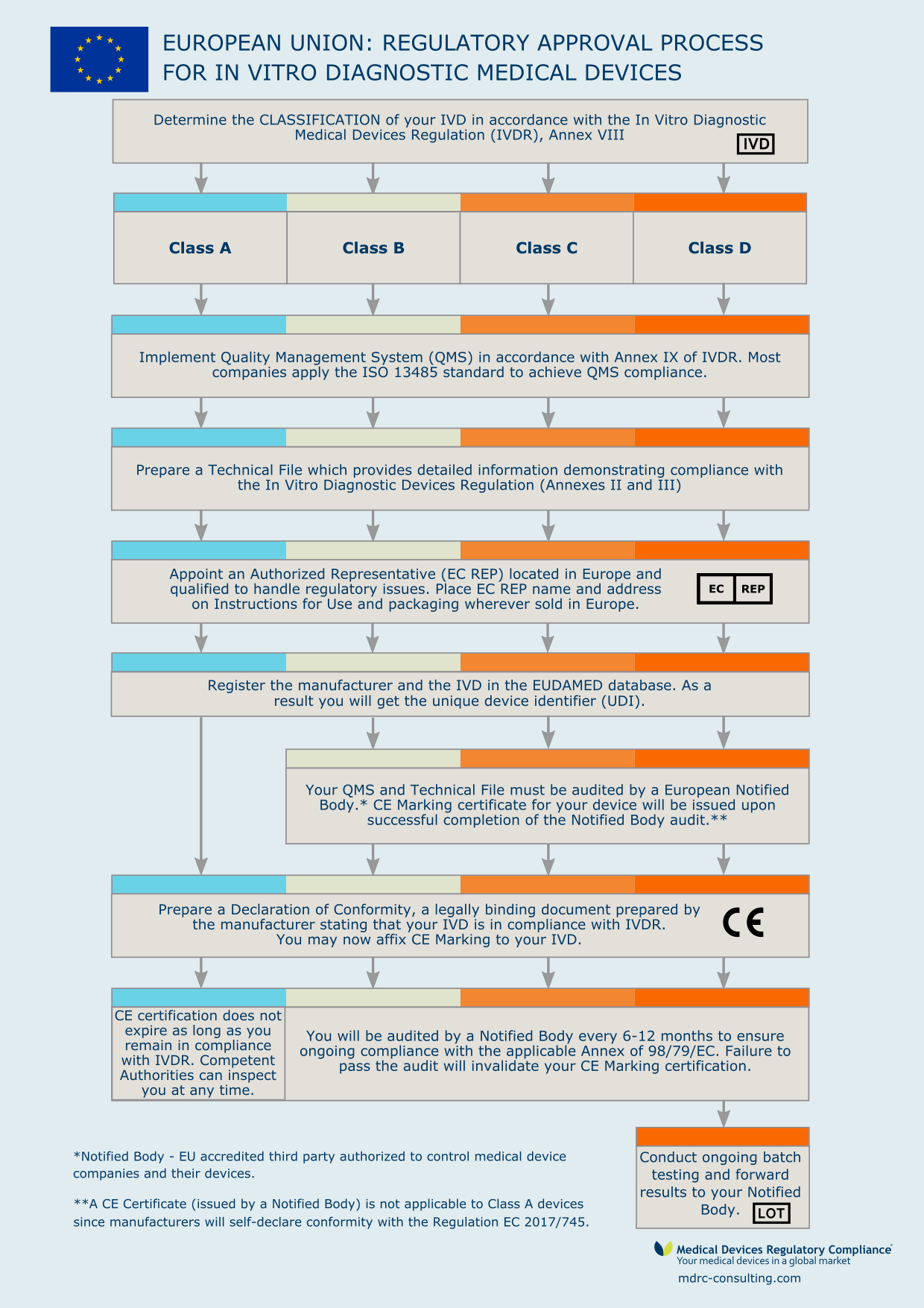
/tuv-rheinland-ivdr-visual-1-en.png)