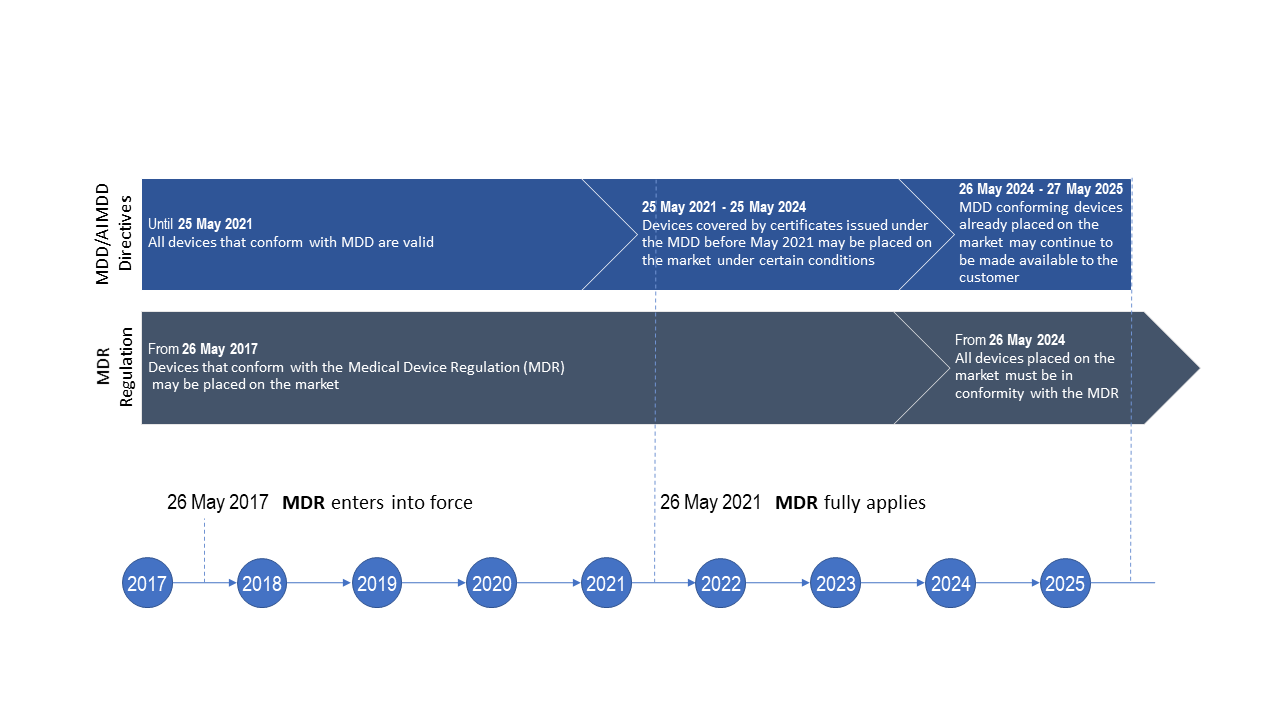
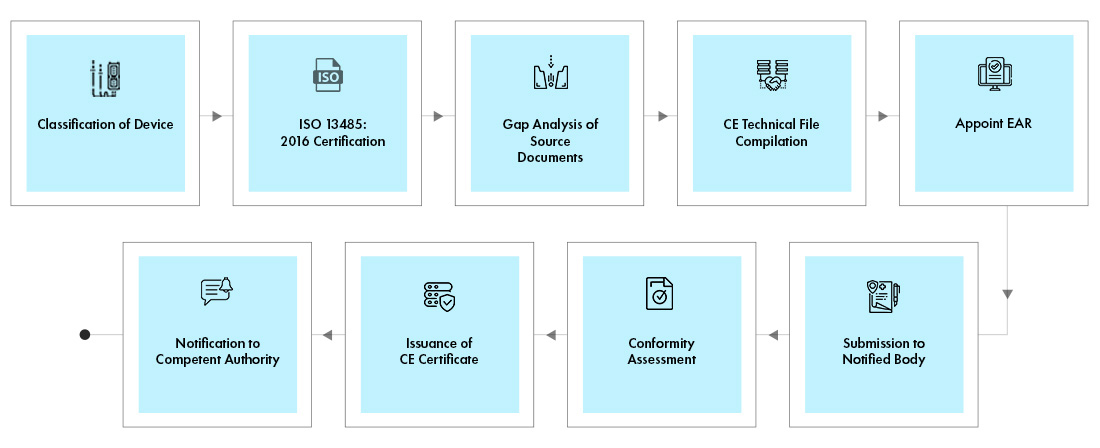
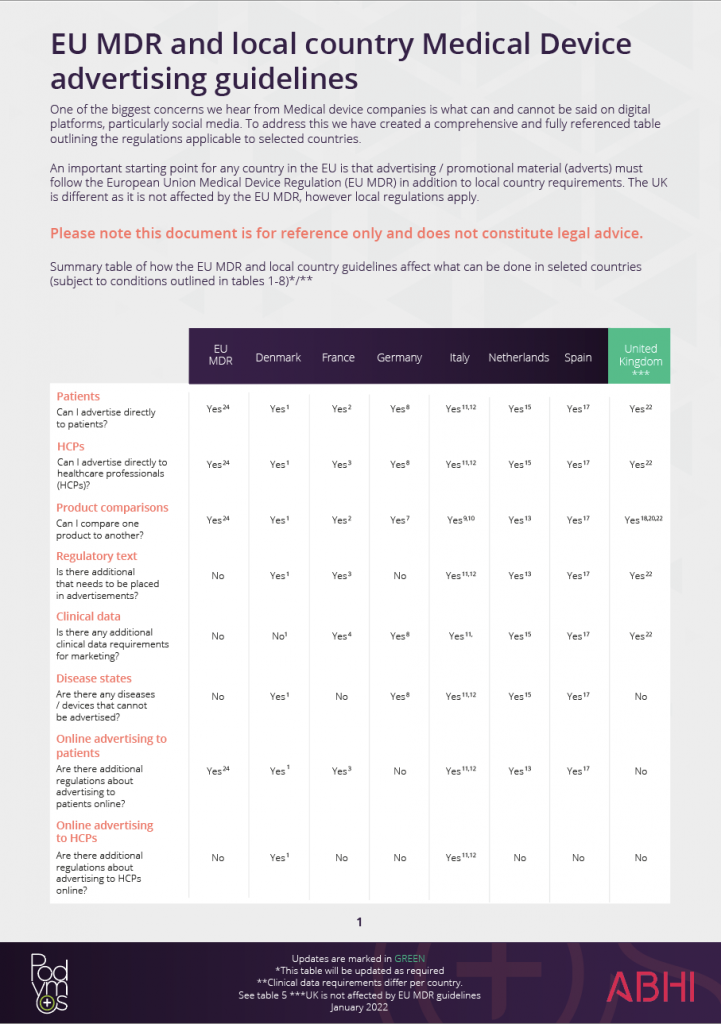
Market access of cell therapies in France: roadmap. *Can be associated... | Download Scientific Diagram

Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention

EUCOPE and France Biotech Medtech/Diagnostic commission have just released a new survey on the Medical Device Regulation (MDR) - France Biotech

Forum EFORT : Registries - MDR/les registres en orthopédie traumatologie - Medical Device Regulation Patient, surgeon and industry | SOFCOT