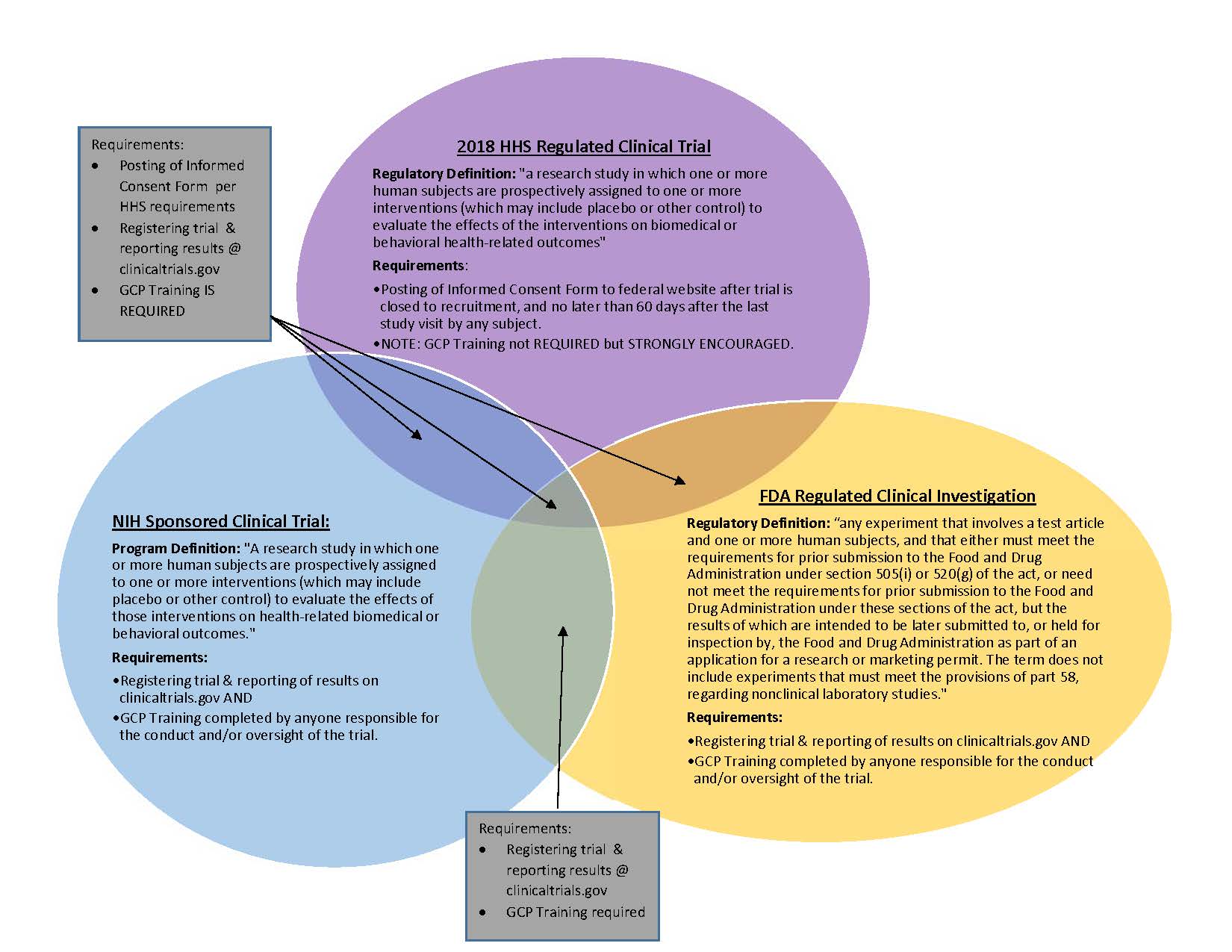
Good Clinical Practice: Pharmaceutical, Biologics, and Medical Device Regulations and Guidance Documents Concise Reference; Volume 2, Guidance: 9780982147689: Medicine & Health Science Books @ Amazon.com

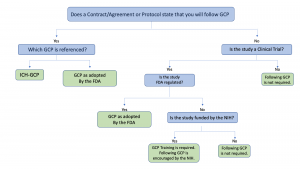
Testing of your GCP knowledge: Test your knowledge of the Clinical Research Regulations and GCP | CRA School | The International Clinical Research Academy

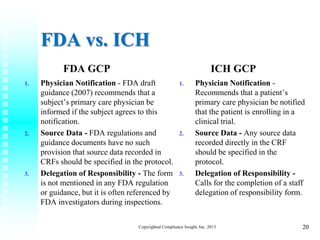
Principles of Good Clinical Practice (GCP) – What is it all about and who is responsible for adherence? GCP and QA All SIAC Call Mar 14, 2008 Munish Mehra, - ppt download

Good Clinical Practice: Pharmaceutical, Biologics, and Medical Device Regulations and Guidance Documents Concise Reference; Volume 2, Guidance: 9780982147689: Medicine & Health Science Books @ Amazon.com