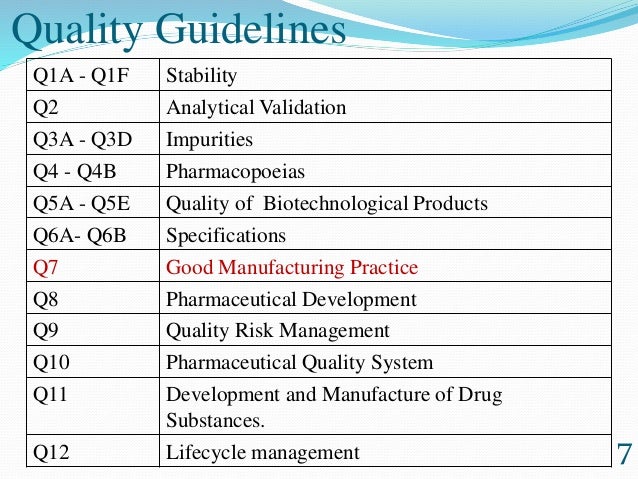
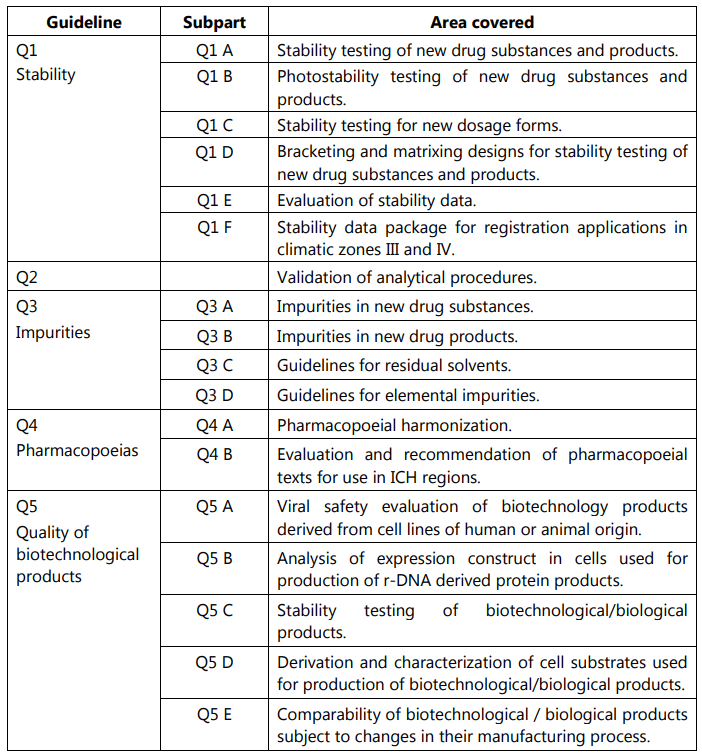
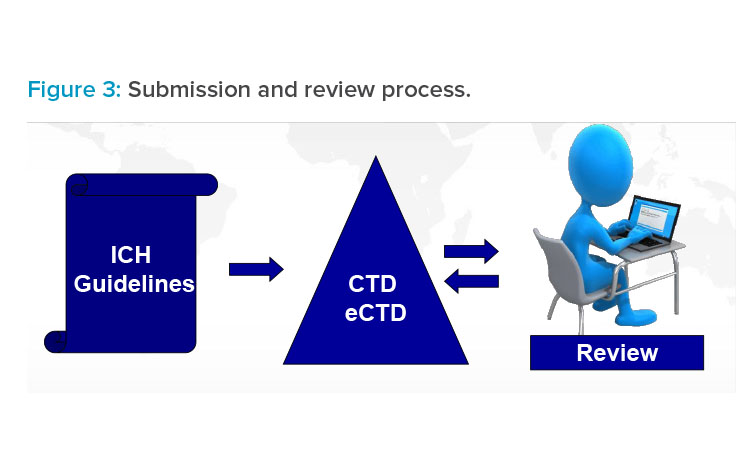
ICH Safety Guidelines from S1A to S12 - TELUGU GMP - Provides GMP Pharmaceutical Guidelines in Telugu.

Compliance with the ICH-GCP Guidelines among the Saudi Health Care Professionals: Should Saudi Arabia Conduct Widespread ICH-GCP Training? | Semantic Scholar

Amazon.fr - Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines - Brody, Tom - Livres
![ROLE OF ICH GUIDELINES FROM ICH-Q1 to ICH-Q14 by Rajashri Ojha[Founder & Director Raaj GPRAC] - YouTube ROLE OF ICH GUIDELINES FROM ICH-Q1 to ICH-Q14 by Rajashri Ojha[Founder & Director Raaj GPRAC] - YouTube](https://i.ytimg.com/vi/KsS_vr312Lo/maxresdefault.jpg)
ROLE OF ICH GUIDELINES FROM ICH-Q1 to ICH-Q14 by Rajashri Ojha[Founder & Director Raaj GPRAC] - YouTube