Devyser's quality management system and fetal diagnostics product approved under the new European IVD Regulation

Europe - MDCG 2022-10 Q&A on the interface between Regulation (EU) 536/2014 on clinical trials for medicinal products for human use (CTR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) - RIS.WORLD
Key Changes in the Regulatory Requirements for In Vitro Diagnostic Devices Marketed in the European Union Under IVDR 2017/746 - Criterion Edge

MedTech Europe Survey Report analysing the availability of In vitro Diagnostic Medical Devices (IVDs) in May 2022 when the new EU IVD Regulation applies - Formiventos
Transition to EU IVD Regulation (EU) 2017/746 and considerations for non-EU regulatory authorities on managing the impact to pro
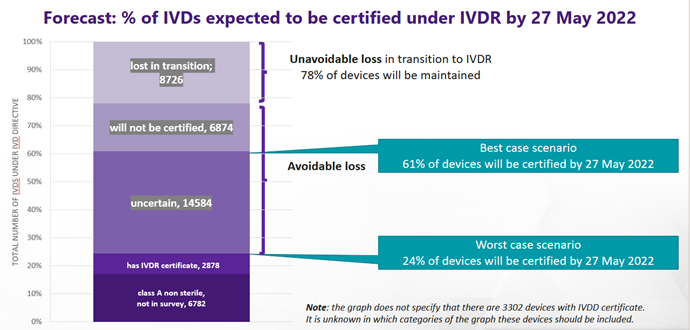


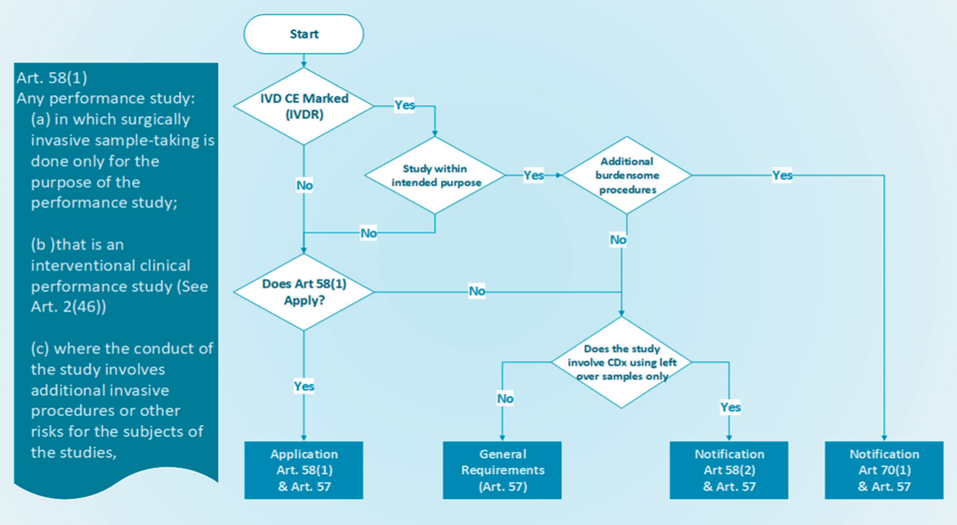







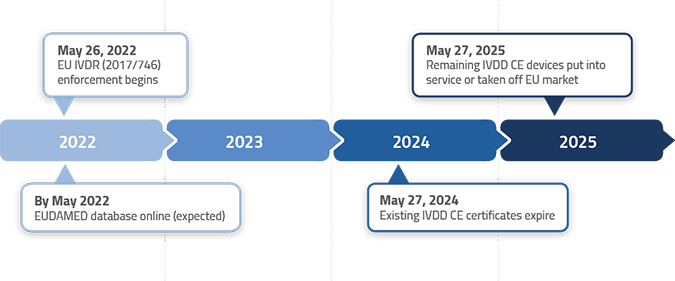
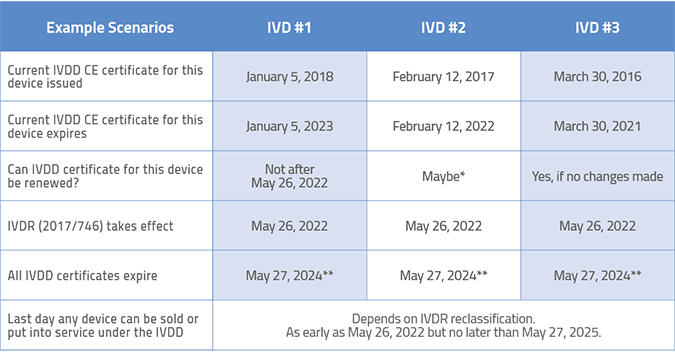
/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)