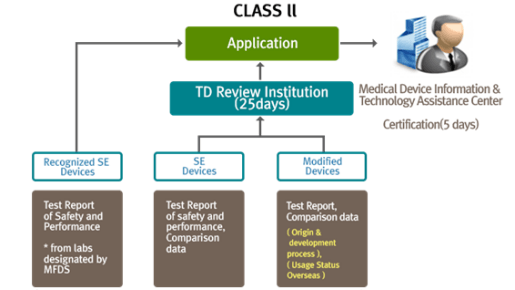
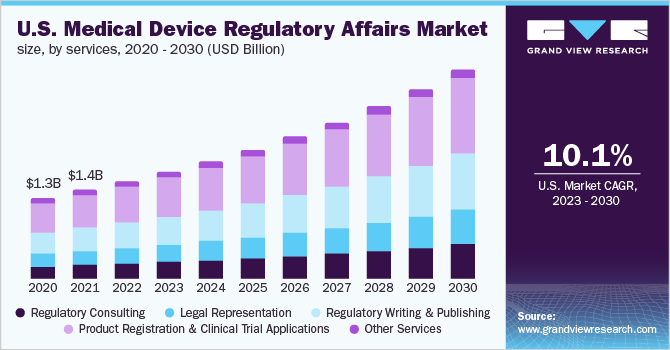
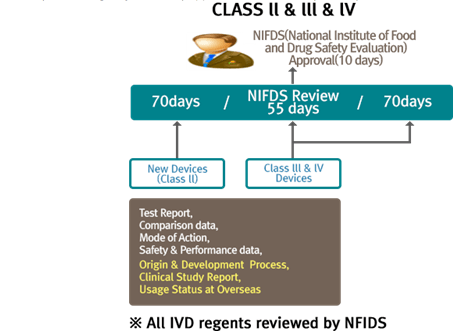
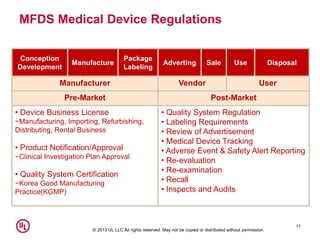
Korean medical device registration: Navigate easily through medical device regulation in South Korea eBook : Kobridge: Amazon.in: Kindle Store
Korea to lead international medical device regulations at Saudi meet this week < Device/ICT < Article - KBR
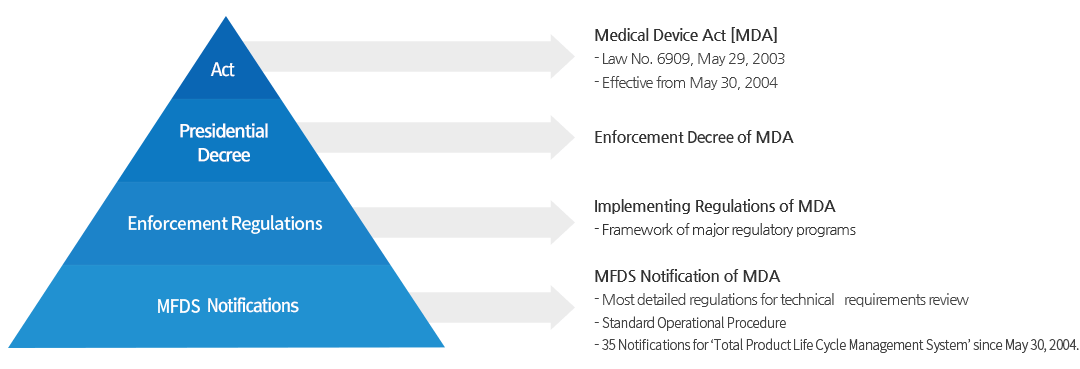
Ministry of Food and Drug Safety>Our Works>Medical Devices>Approval Process | Ministry of Food and Drug Safety
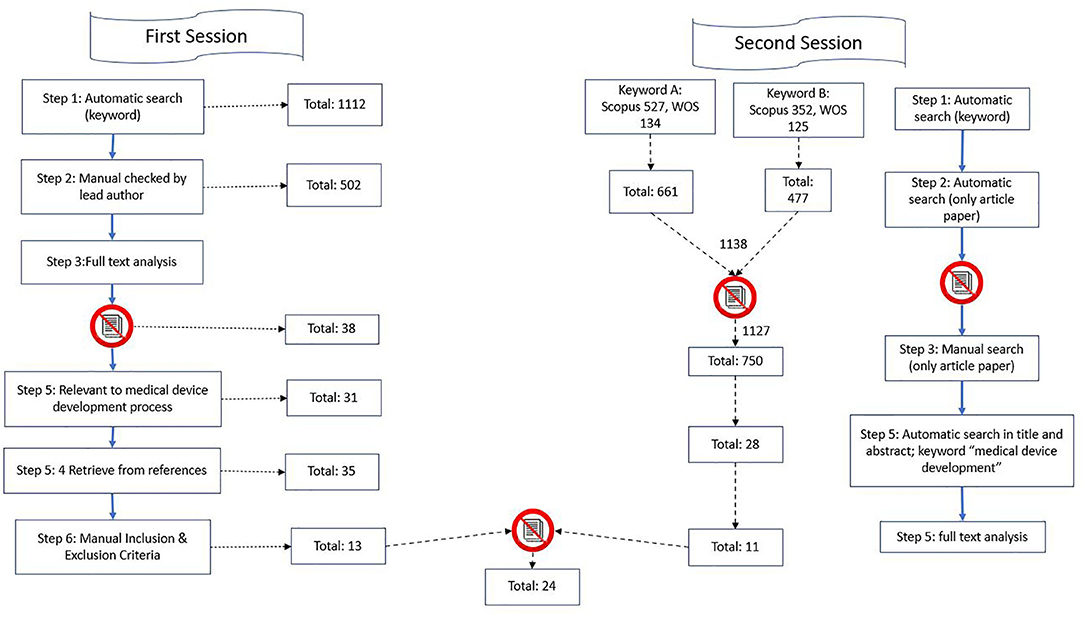
Frontiers | Medical Device Development Process, and Associated Risks and Legislative Aspects-Systematic Review
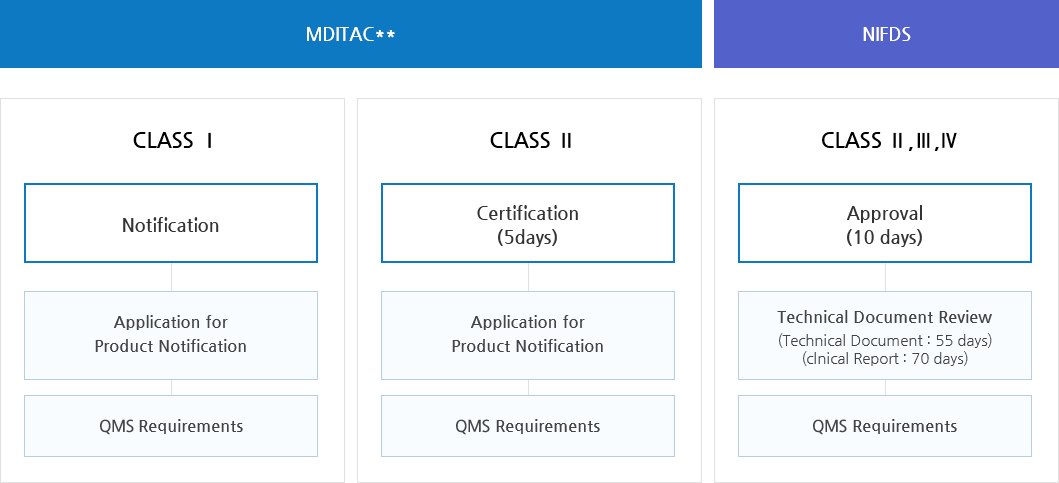
Ministry of Food and Drug Safety>Our Works>Medical Devices>Approval Process | Ministry of Food and Drug Safety
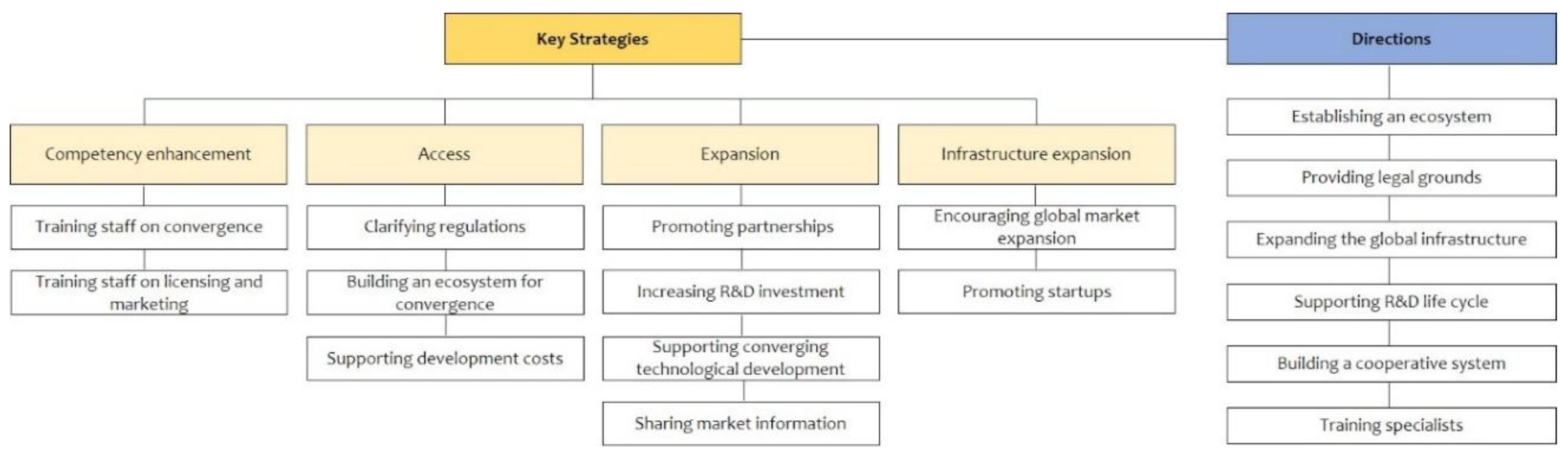
IJERPH | Free Full-Text | Strategies for Promoting the Medical Device Industry in Korea: An Analytical Hierarchy Process Analysis


/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)