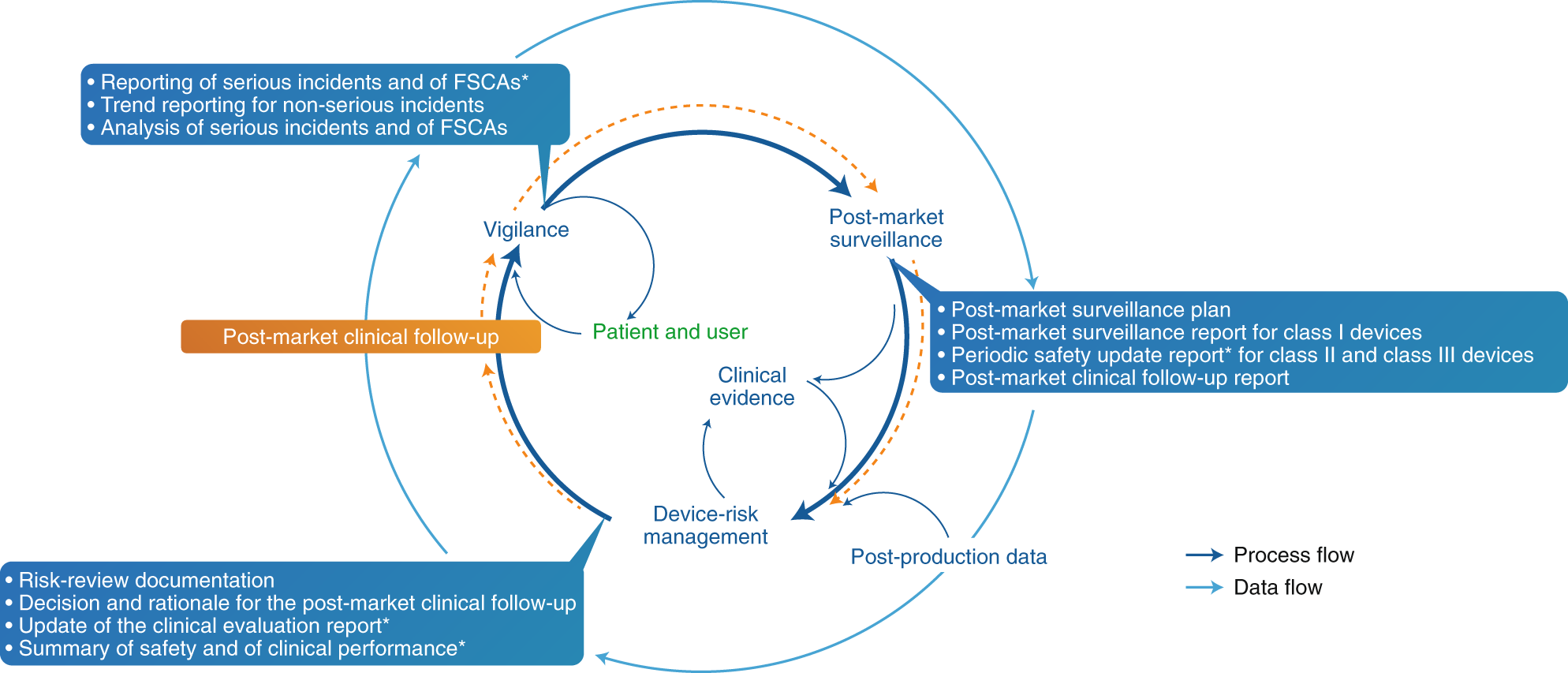
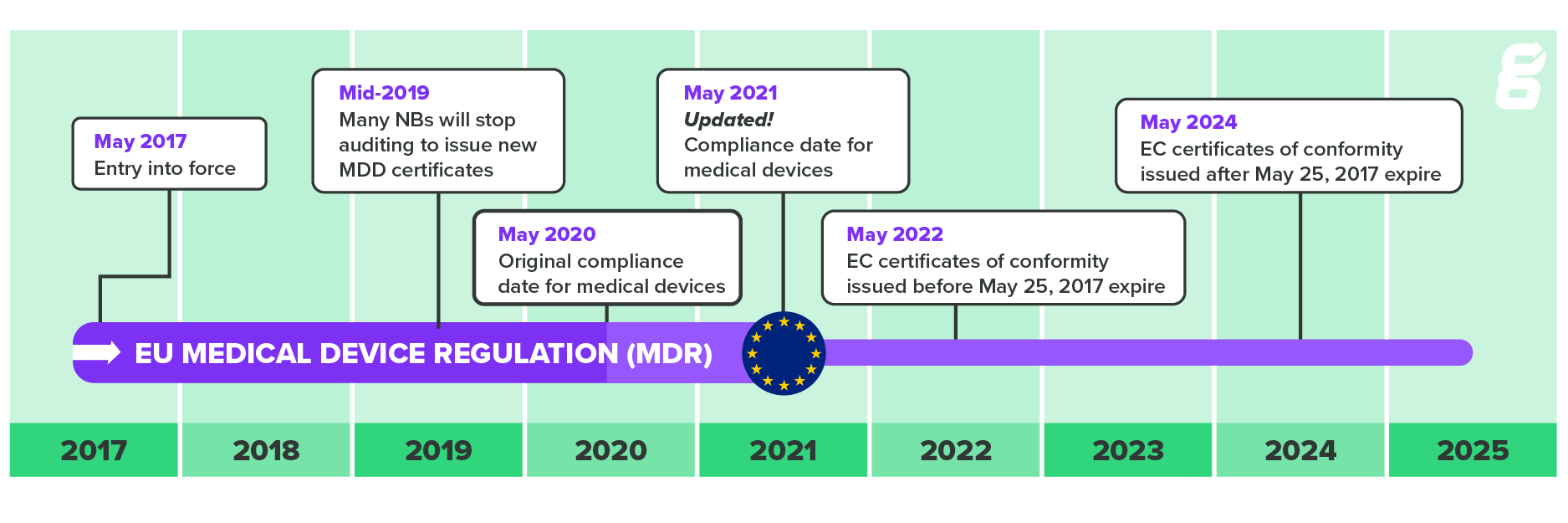
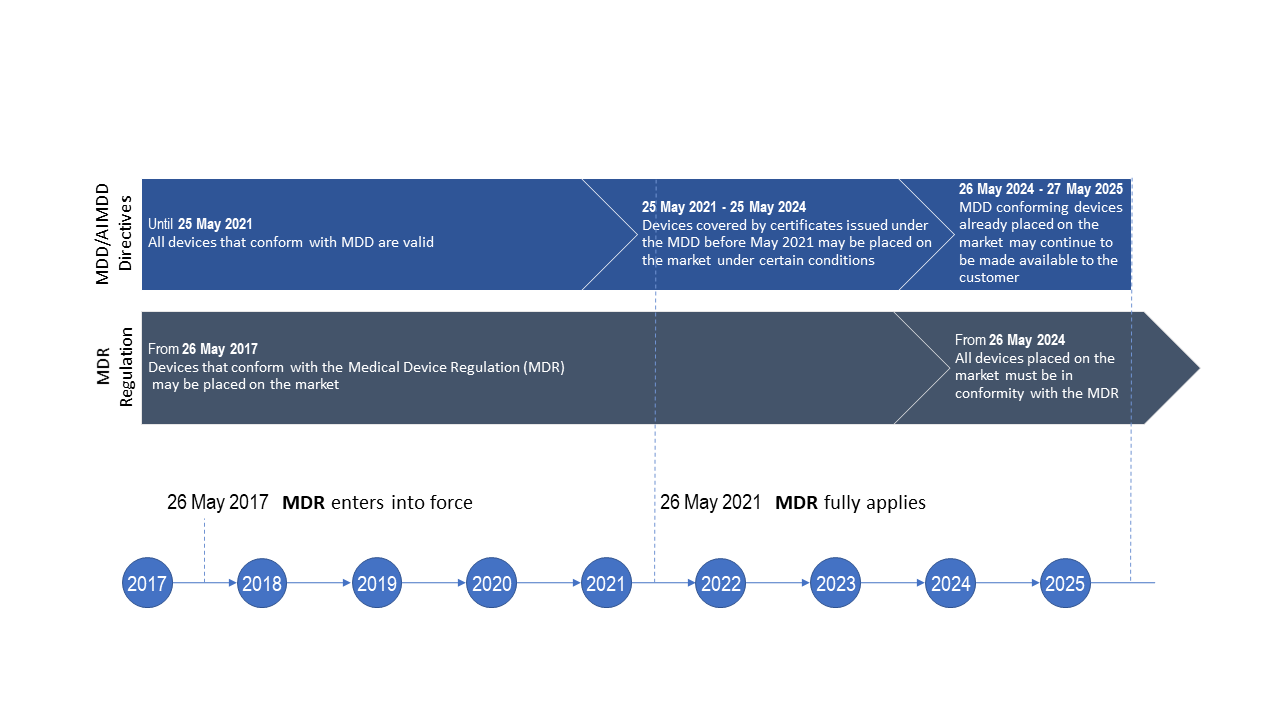
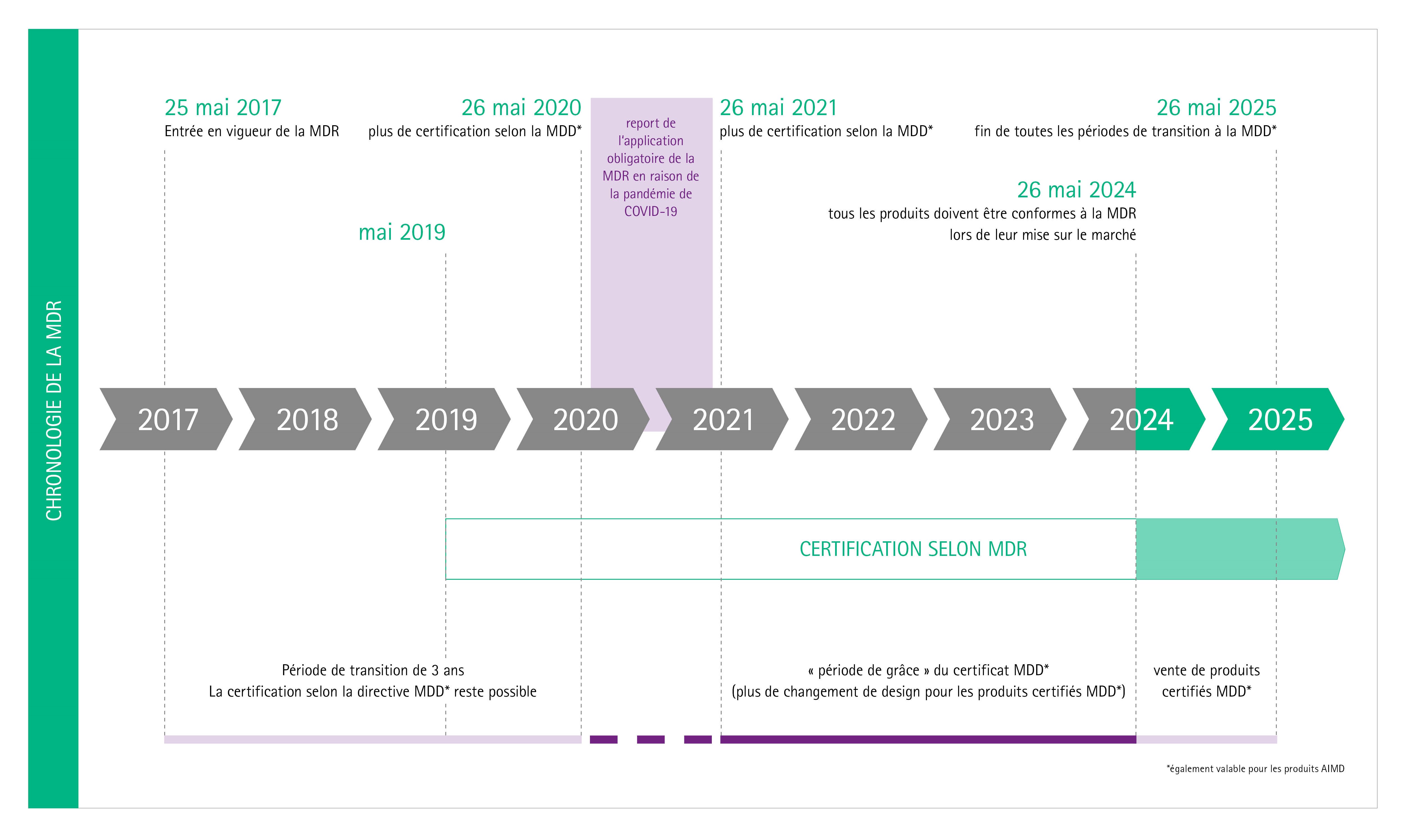
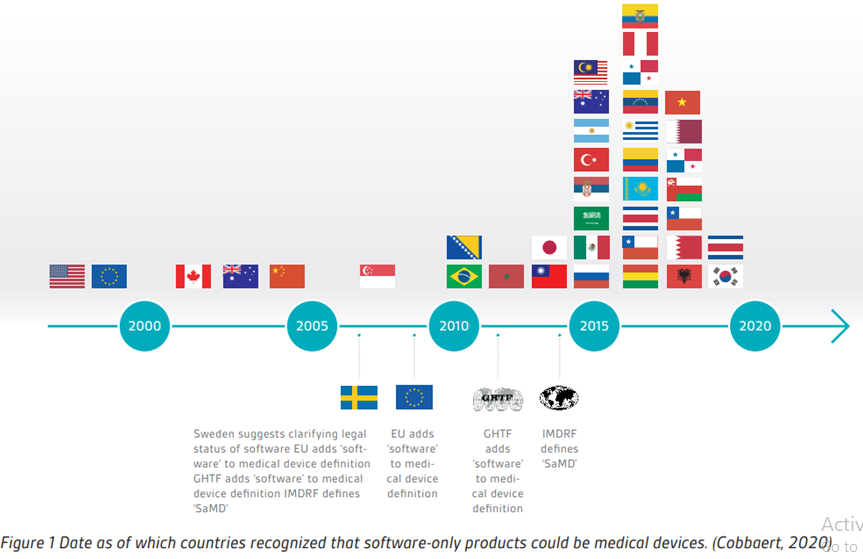
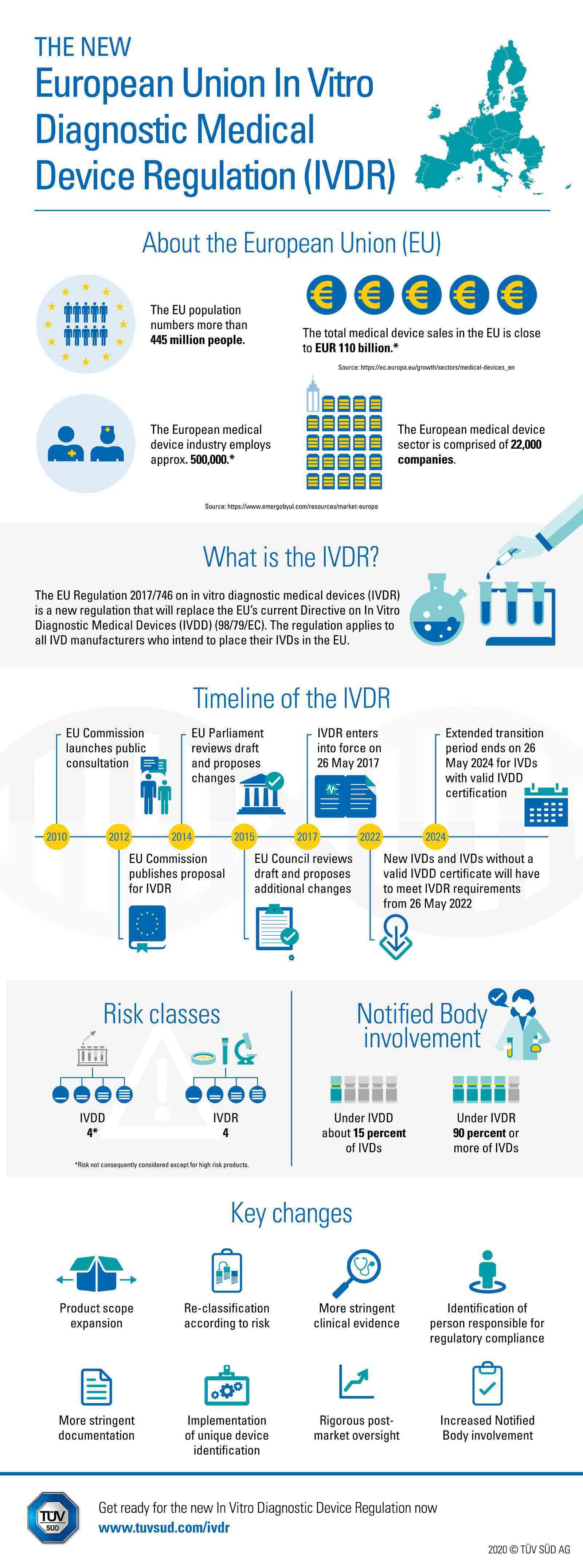
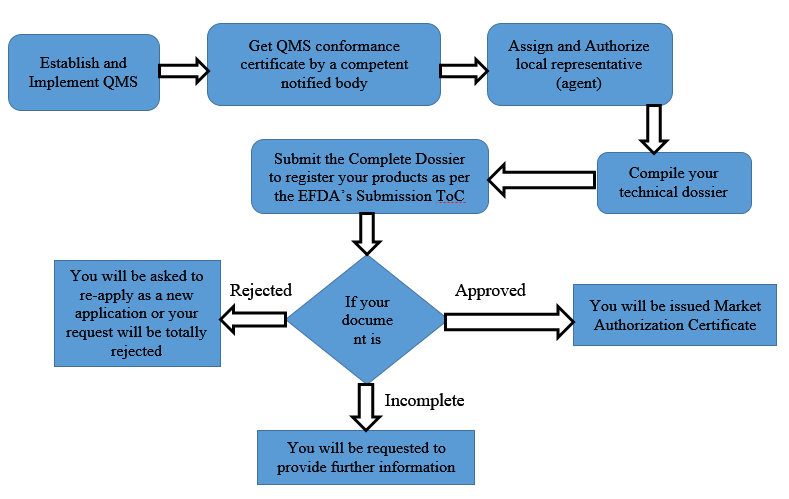
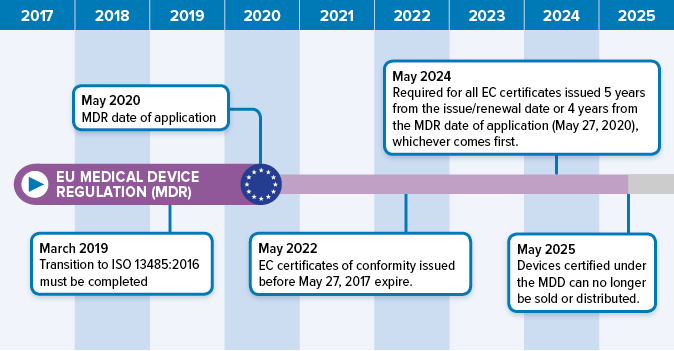
BVMed on Twitter: "For today's "Day of Application" of the EU Medical Device Regulation #MDR, we offer various infographics on the timeline, certificates, building sites and Notified Bodies. https://t.co/Eu9Am4ZCWy #MDReady #Medtech https://t.co ...

Think Quick, Act Fast! What U.S. Medical Device Manufacturers Need to Know About the EU Medical Device Regulation and its Approaching Deadline – Ninety-Nine Percent
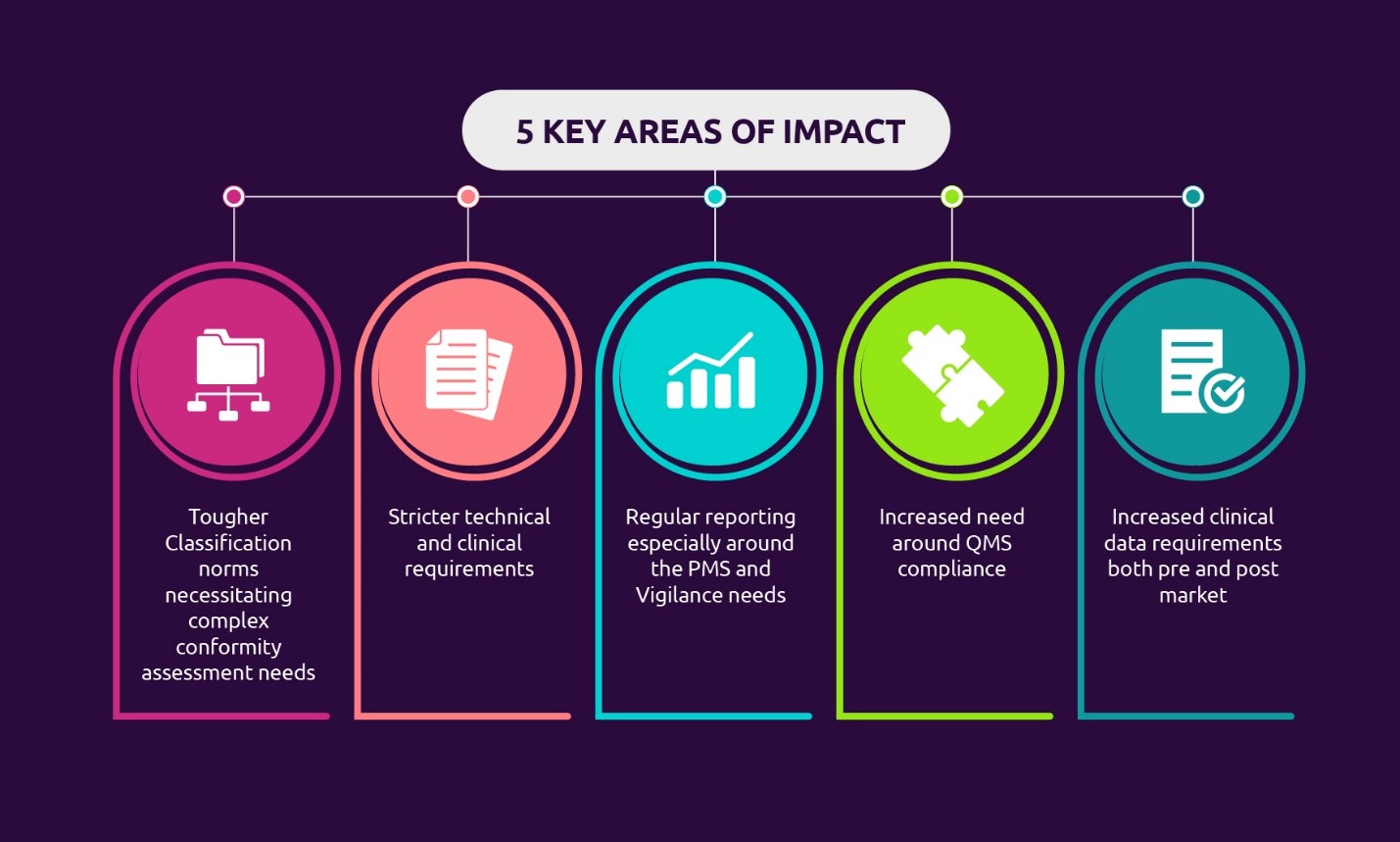
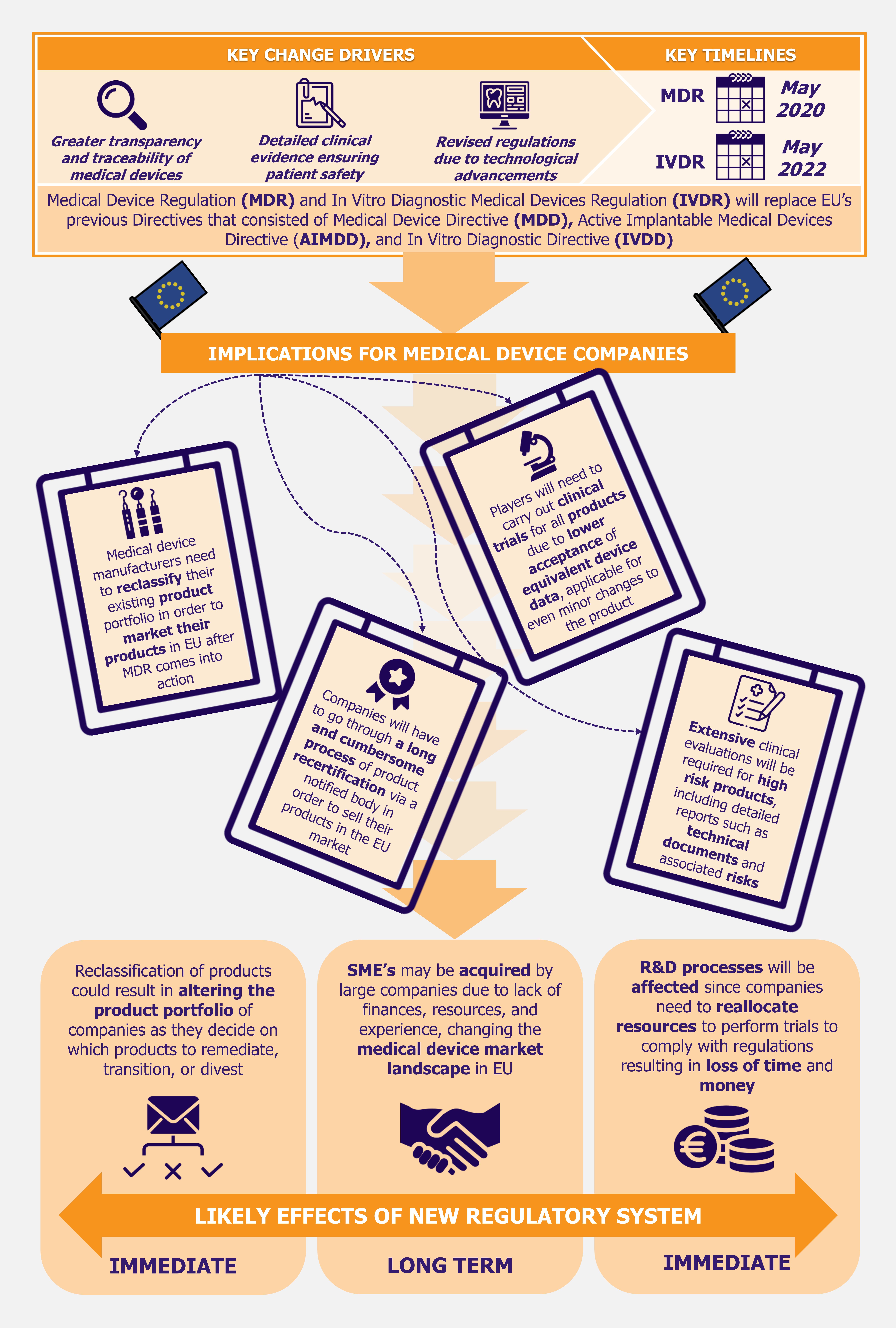
Remediation implications for medical device manufacturers in changing regulatory landscape | Capgemini