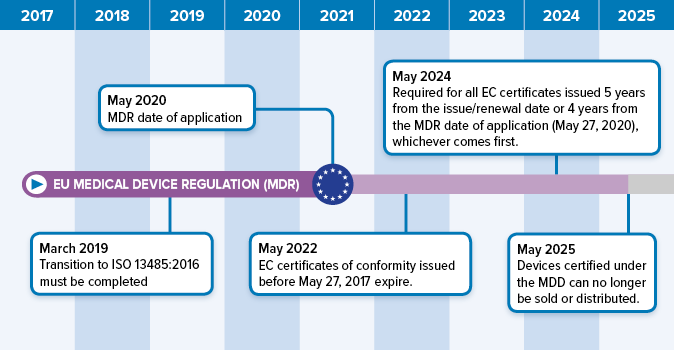
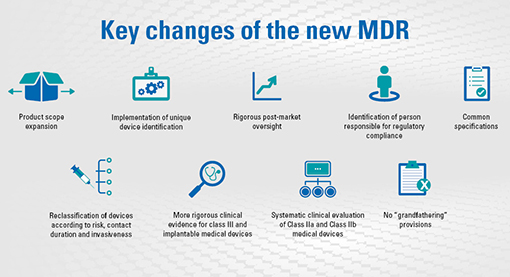
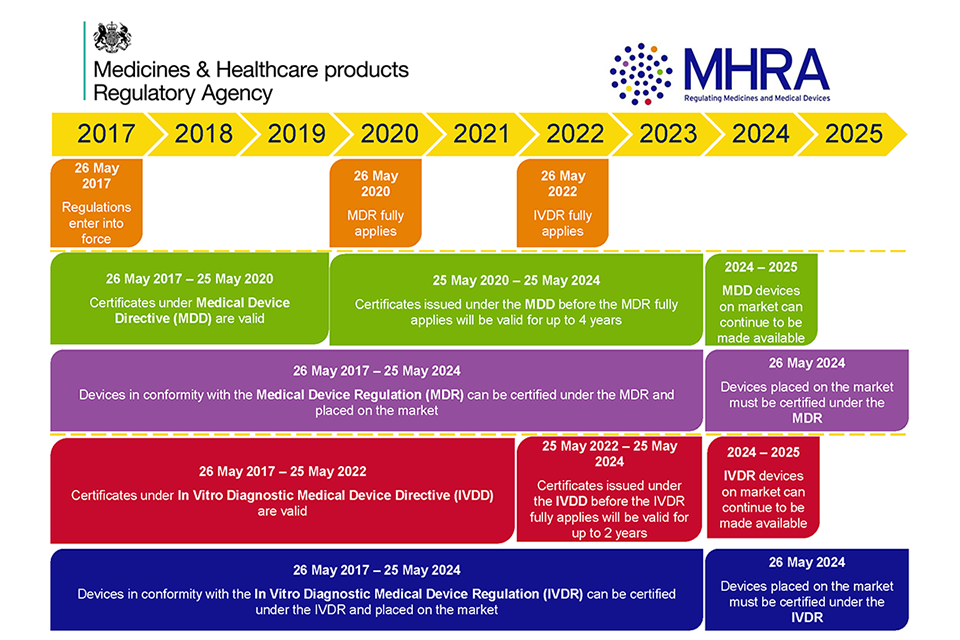
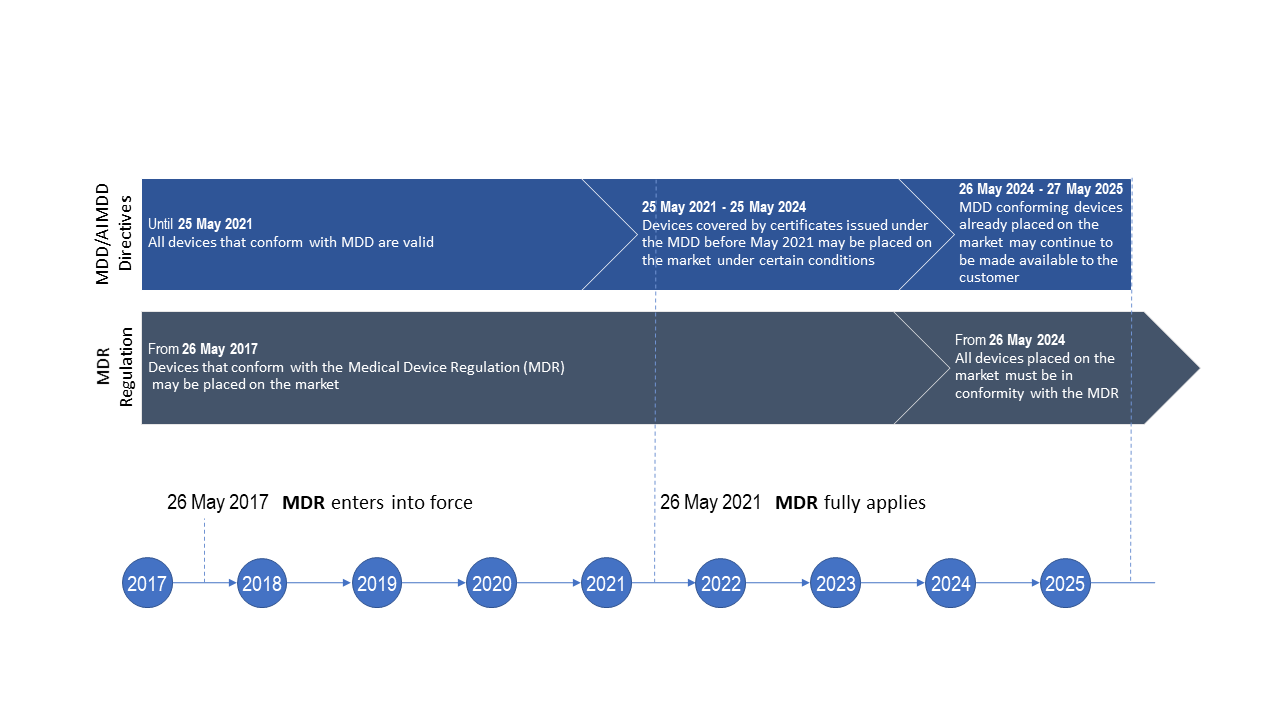
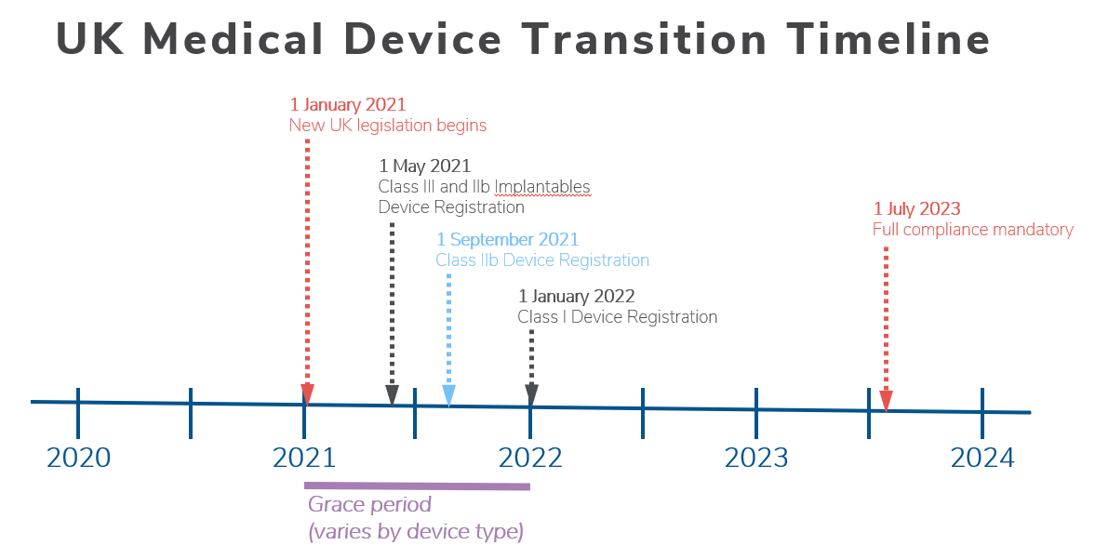
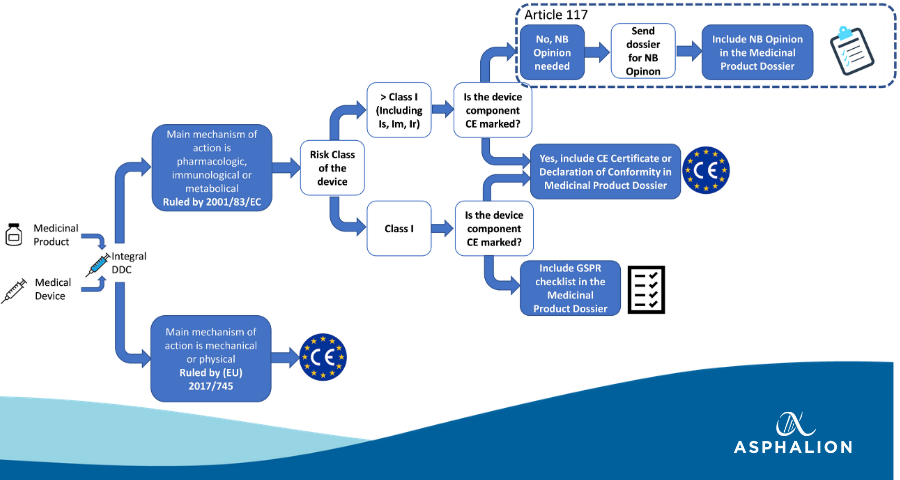
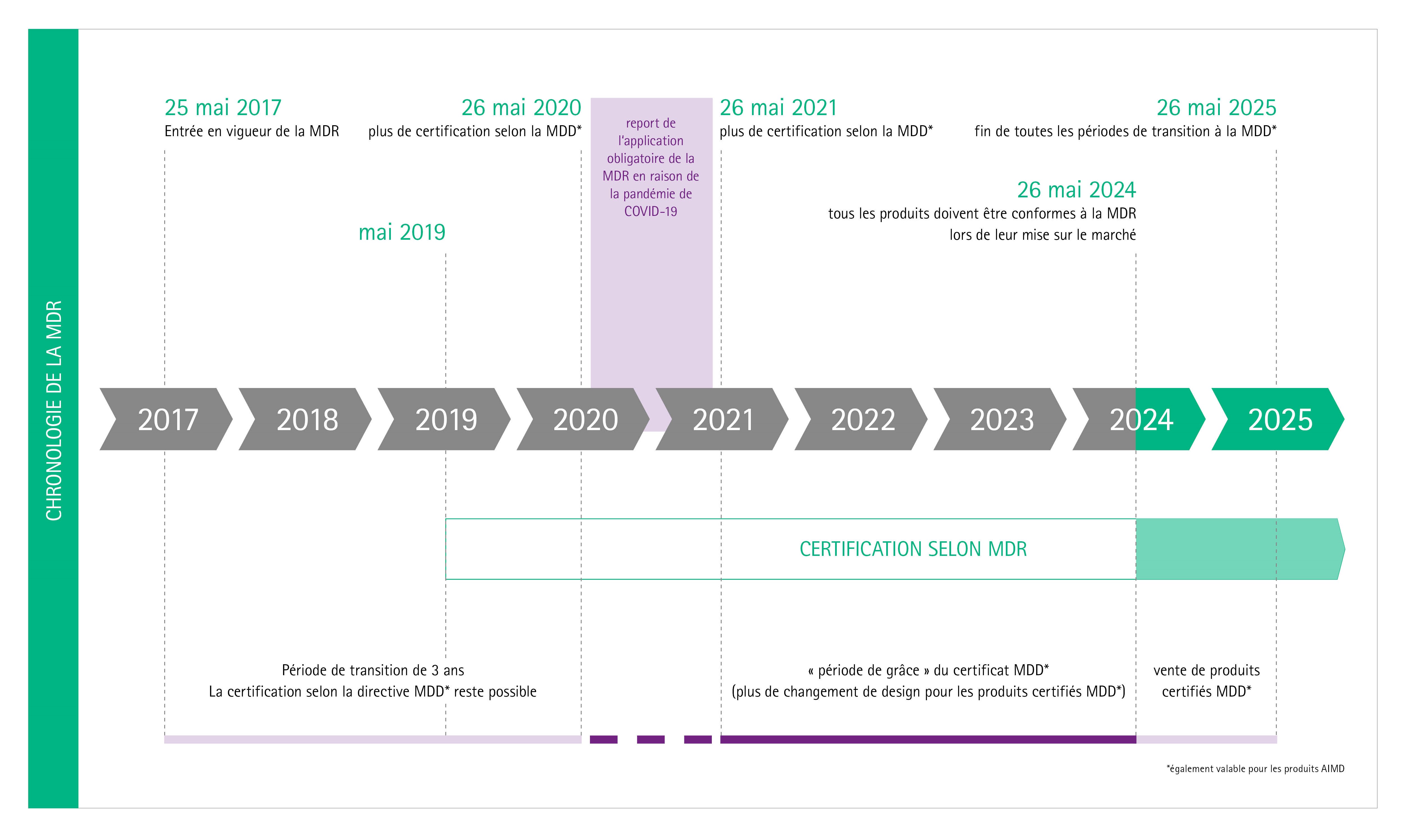
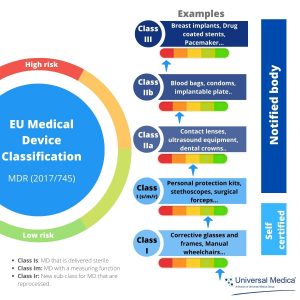
Prosthesis | Free Full-Text | Medical Device Regulation from a Health Service Provider's Perspective

QT Analysis: 5 Pivotal Regulation Updates for Medical Devices that You Need to Know from PFDA – September 2021
Hey, European Commission, it's time to copy-paste Australian regulation! - Software in Medical Devices, by MD101 Consulting
![2021 MFDS-ACRS Conference] Drug Device Combination Products in the EU &The Impact of the New EU MDR - YouTube 2021 MFDS-ACRS Conference] Drug Device Combination Products in the EU &The Impact of the New EU MDR - YouTube](https://i.ytimg.com/vi/1-rRZ_0MkiA/maxresdefault.jpg)