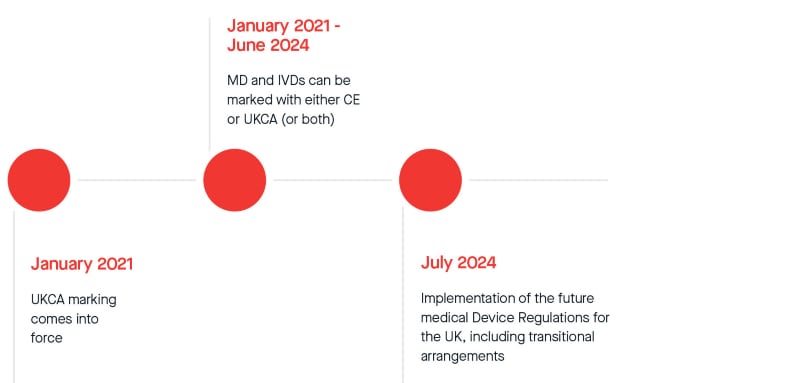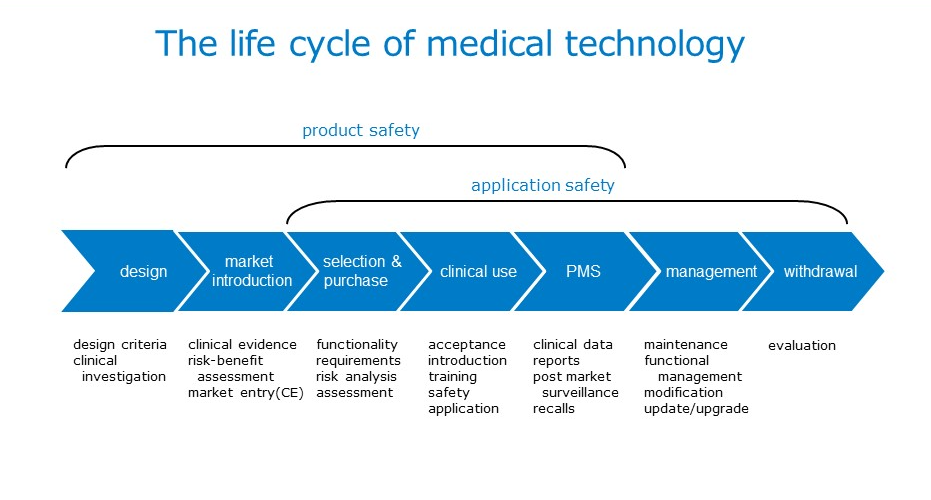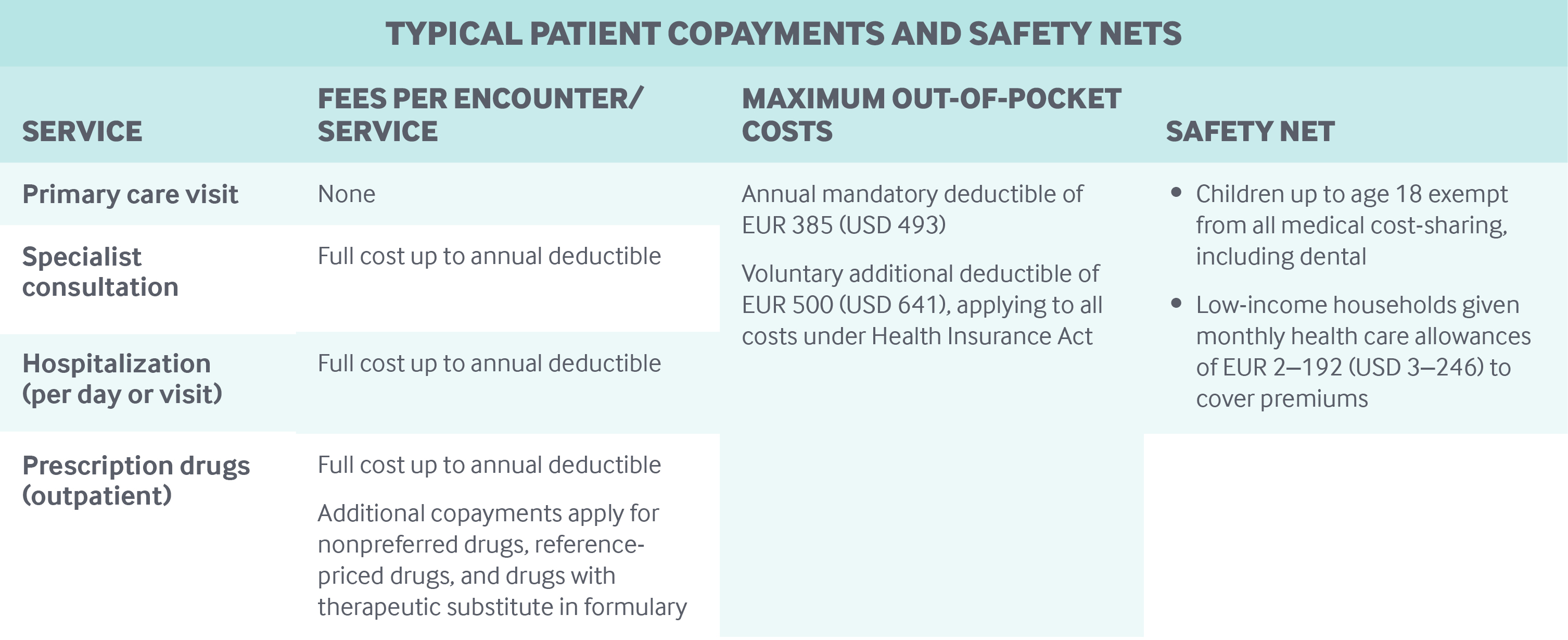
Netherlands Healthcare (Pharma and Medical Devices) Market Analysis, Regulatory, Reimbursement and Competitive Landscape
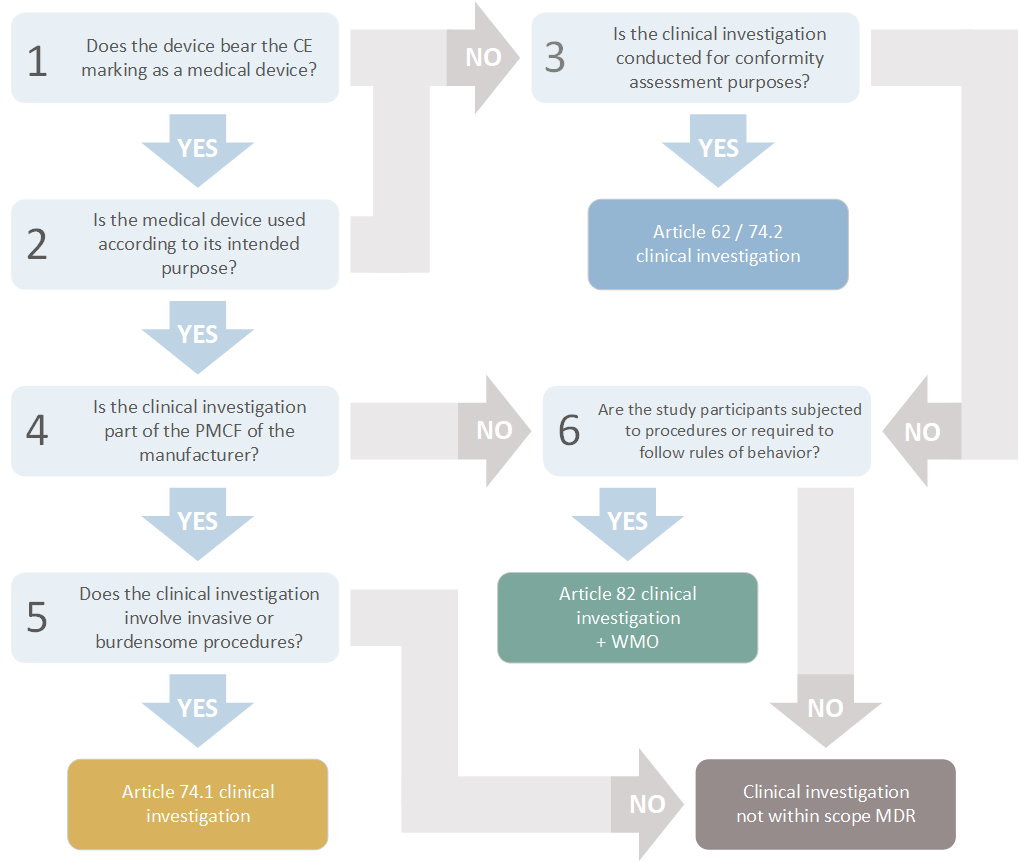
Clinical investigations: definition and framework | Investigators | The Central Committee on Research Involving Human Subjects
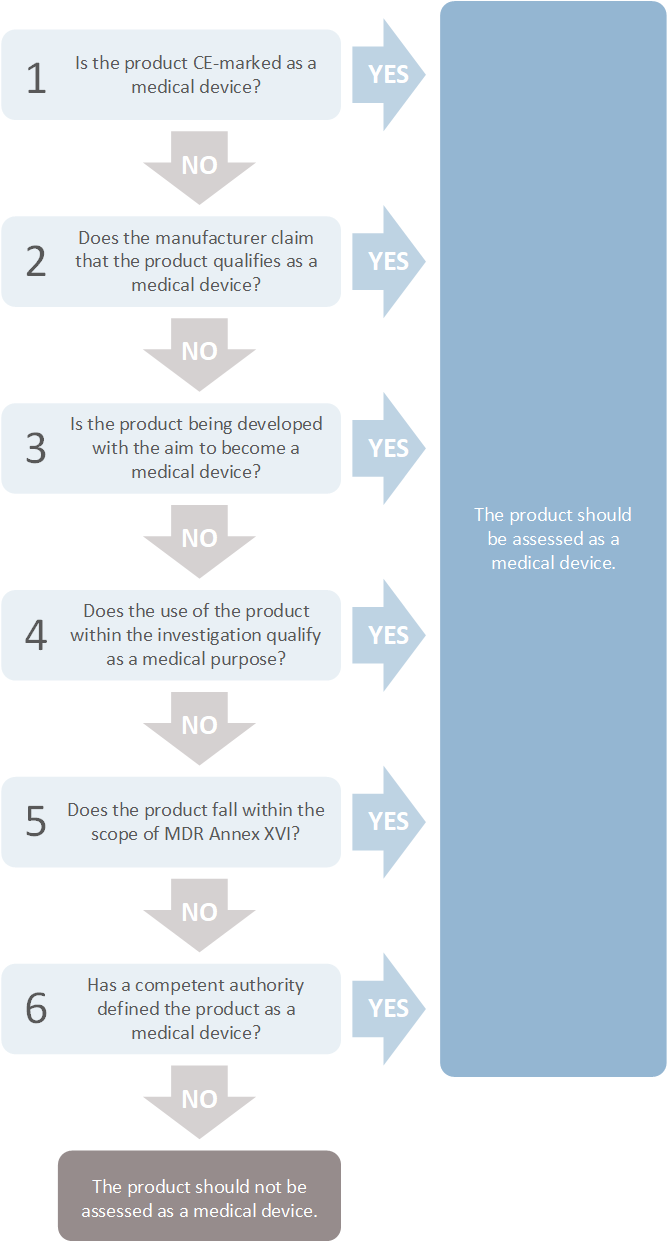
What is a medical device? | Investigators | The Central Committee on Research Involving Human Subjects

The Netherlands as gateway to Europe following the new Medical Device Regulations (MDR) in May 2021 - YouTube

Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention