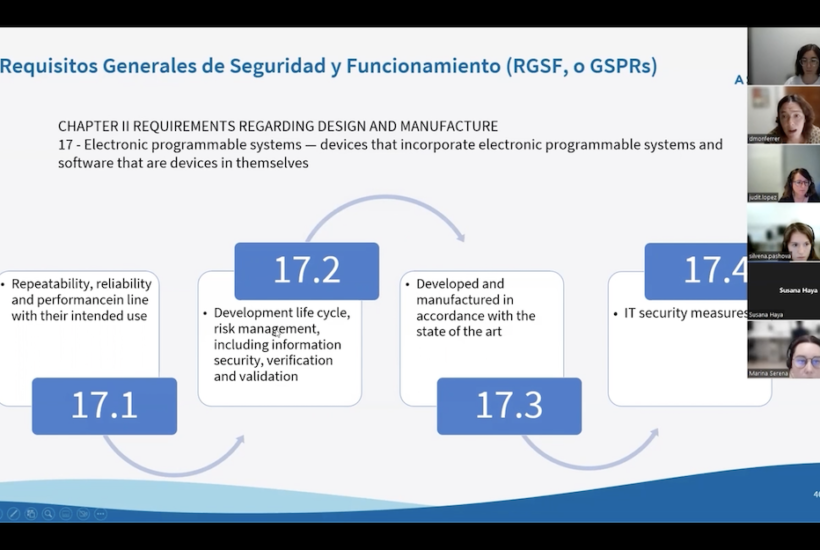
Hey, European Commission, it's time to copy-paste Australian regulation! - Software in Medical Devices, by MD101 Consulting
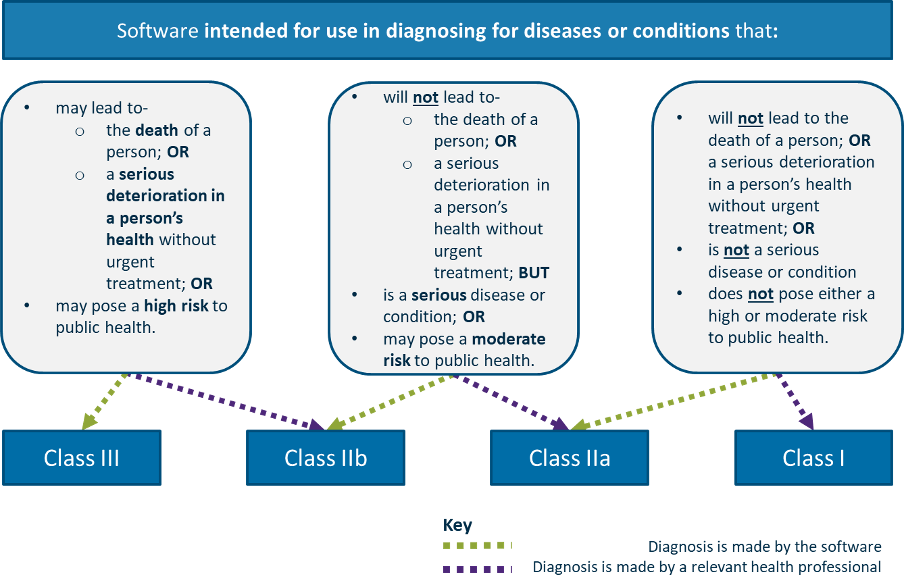
Medical Software Development according to Medical Device Regulation (MDR) – part 1: Software qualification and classification

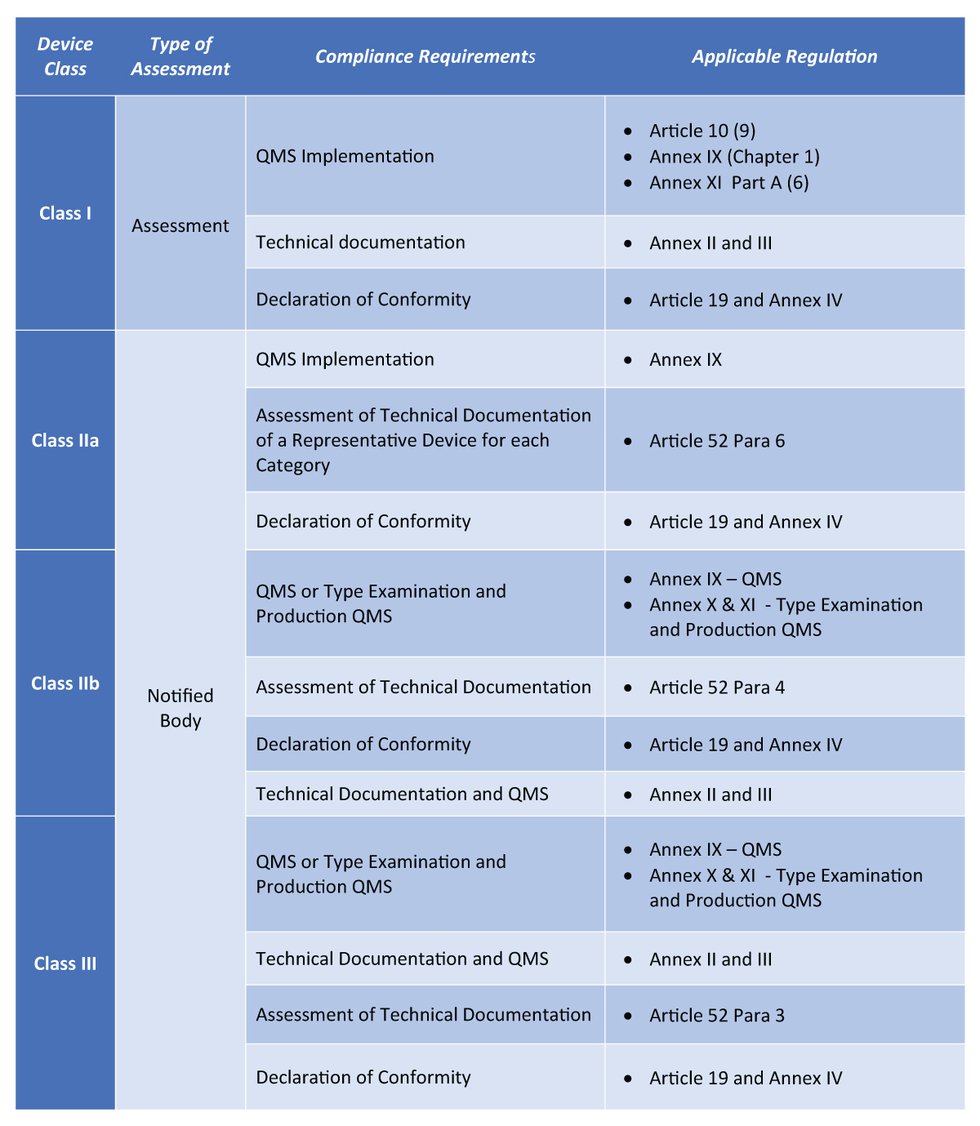
Classification of Software as a Medical device under Medical Device Regulation (European Union) - Kvalito
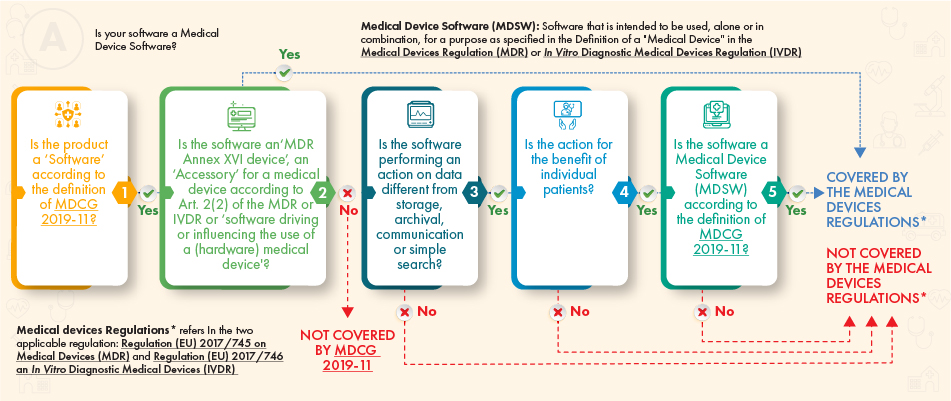
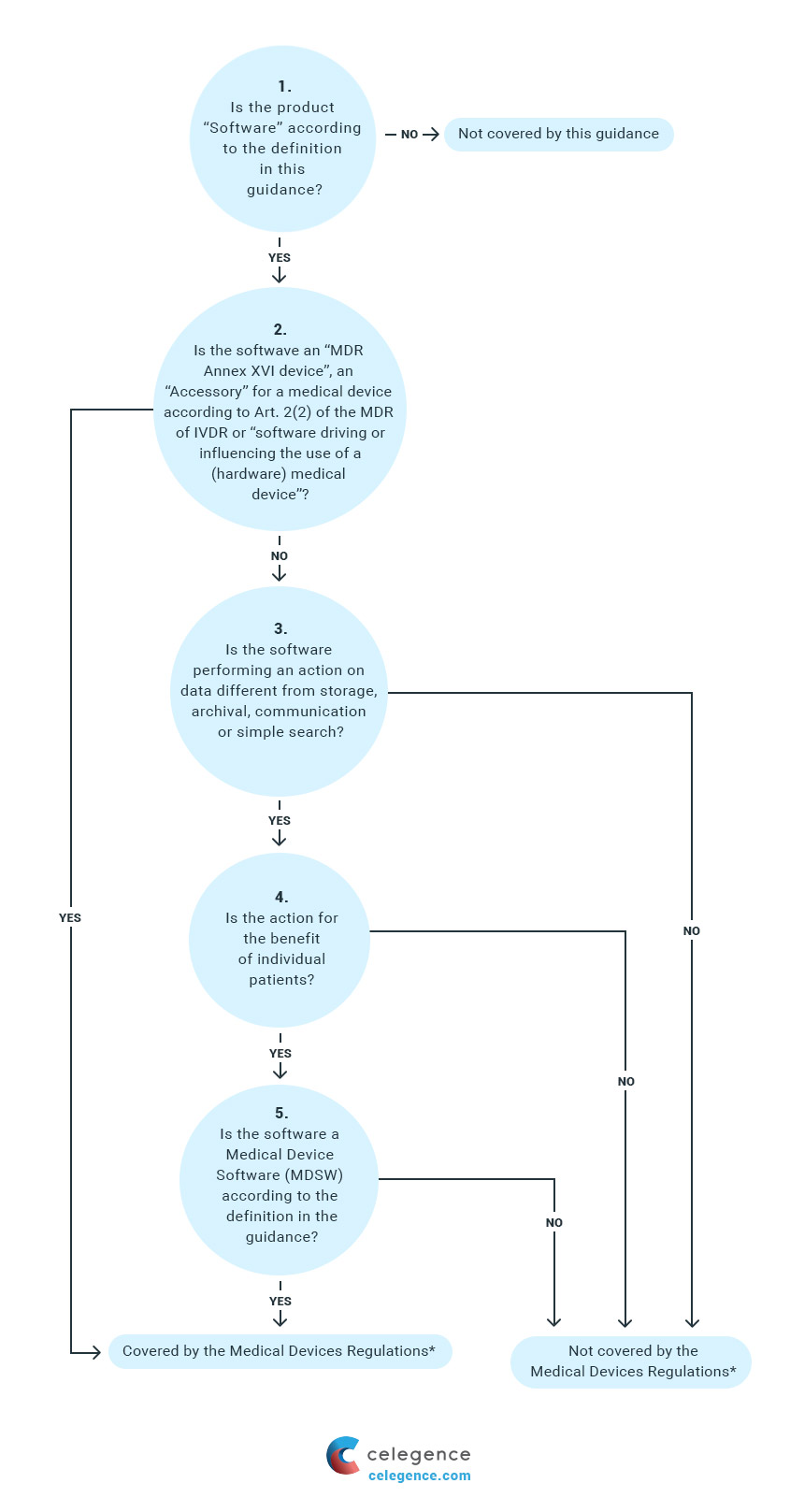
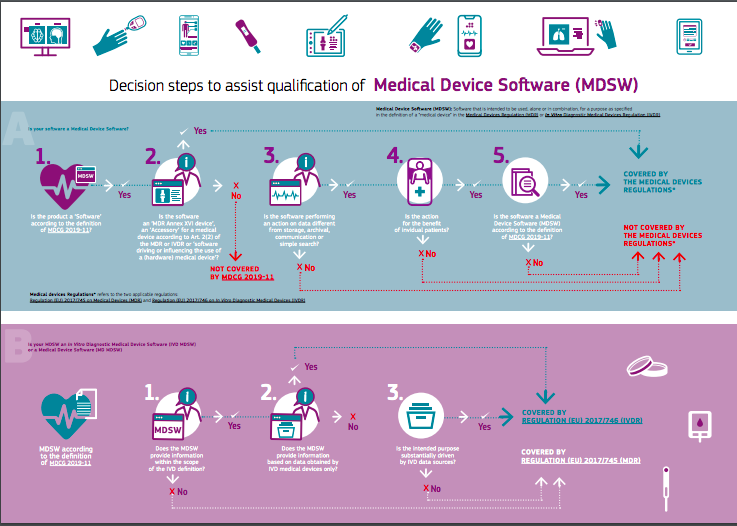
MDCG Guidance for Medical Device Software | Freyr - Global Regulatory Solutions and Services Company
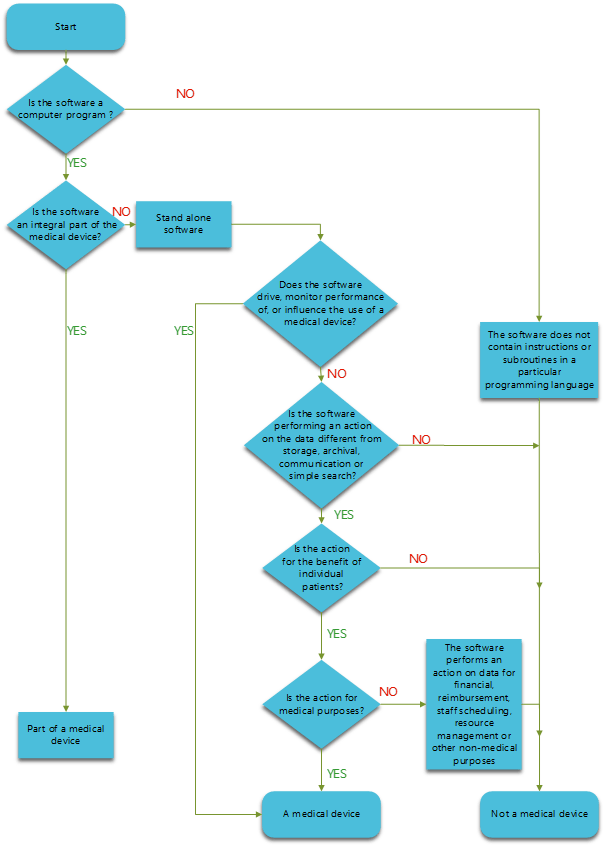
Classification of Software as a Medical device under Medical Device Regulation (European Union) - Kvalito

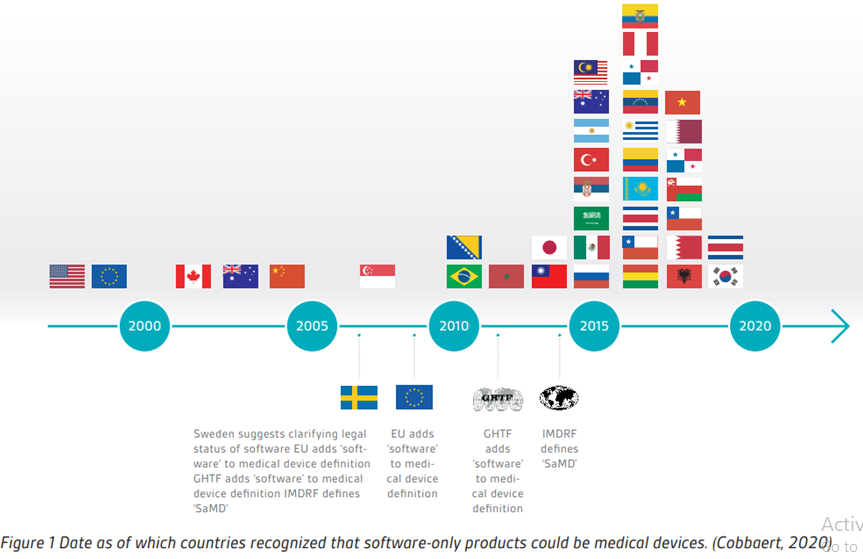
software as a medical device in light of the new MDR: 12 points of consideration for developers (part 2 of 3) • turing law
How will Europe's new device regulations affect medical software? - Medical Technology | Issue 40 | June 2021