Martina Bernini on Twitter: "Just completed a free Mini-Course on New Medical Device Regulation (MDR 2017/745). Thanks to @EasyMedDevice for this interesting course. https://t.co/QChznt171K https://t.co/yCo5XiqI3b" / Twitter

Global Institute of Regulatory Affairs - Hurry Up, Admissions Open!!!! Limited seats are available... Register now at GIRA to become an expert in regulatory affairs, We offer an online Medical Device Regulation

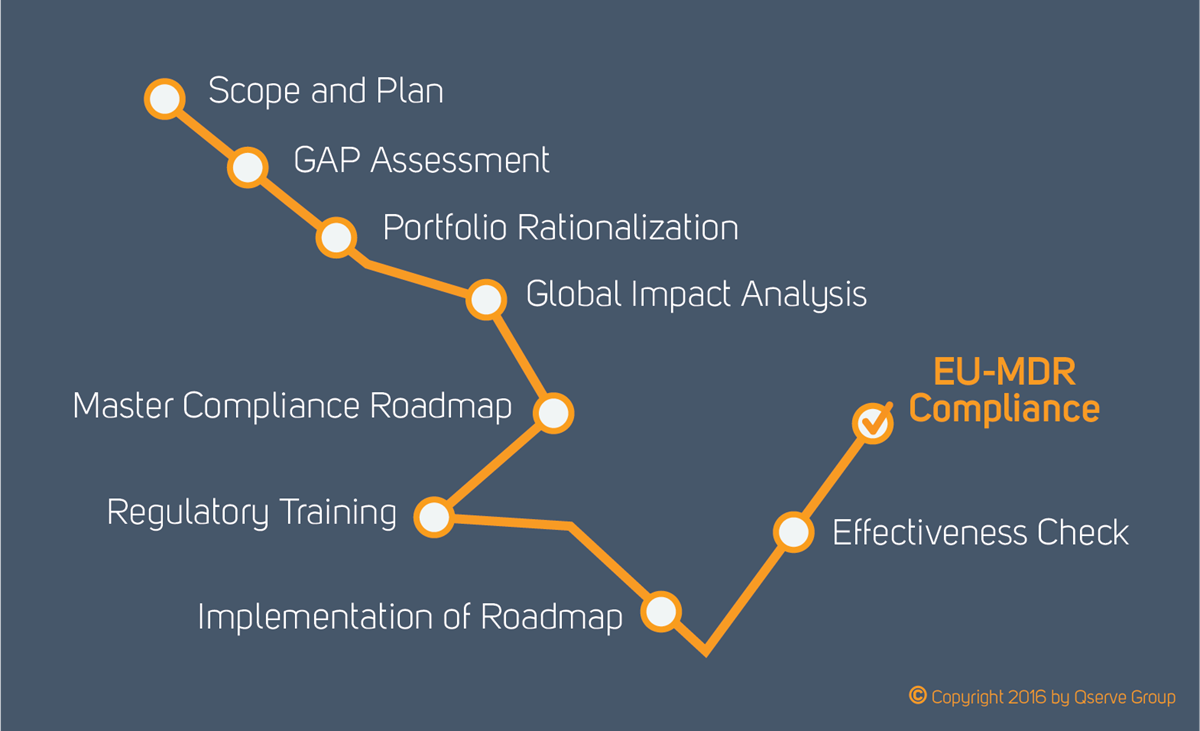
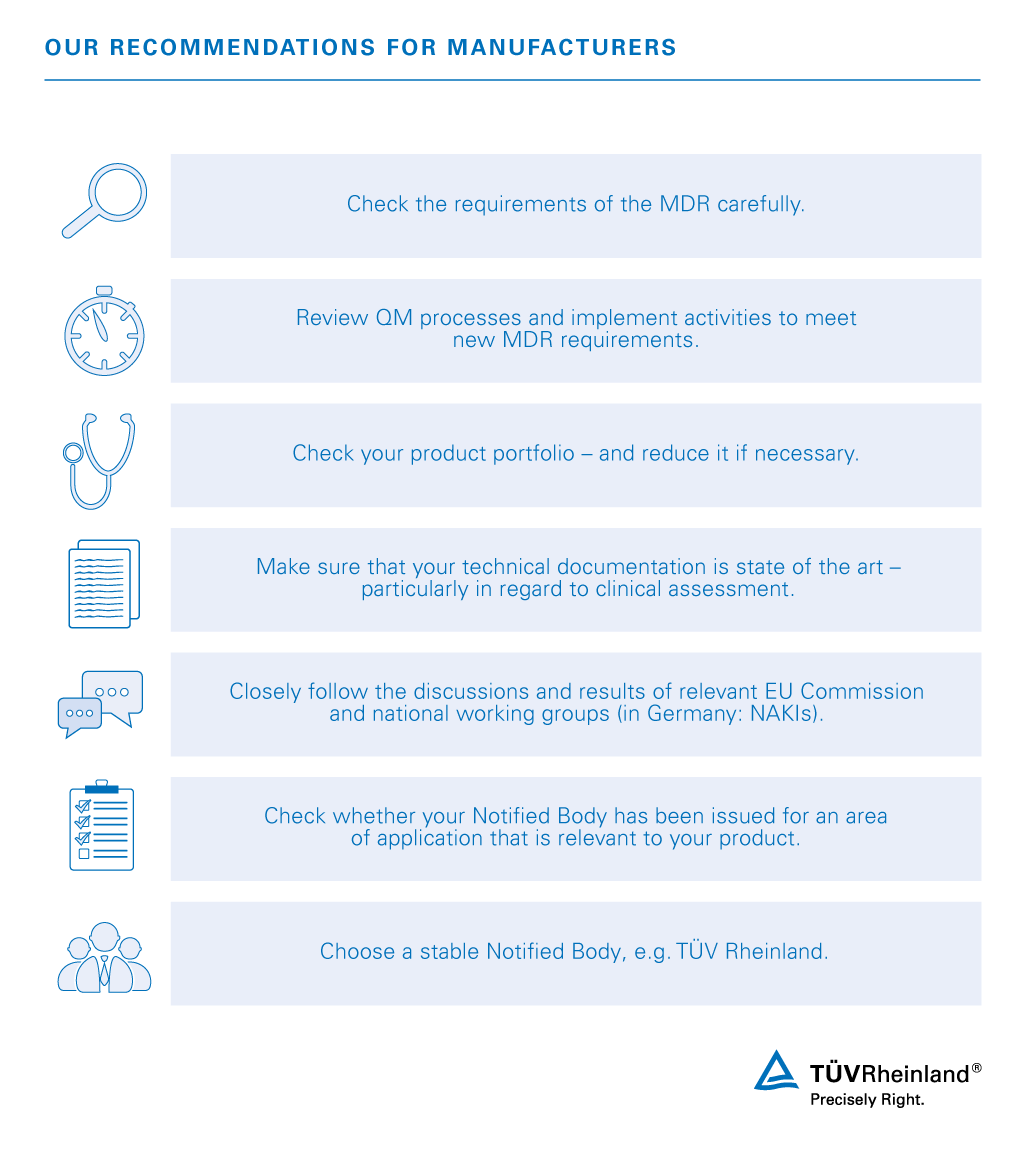
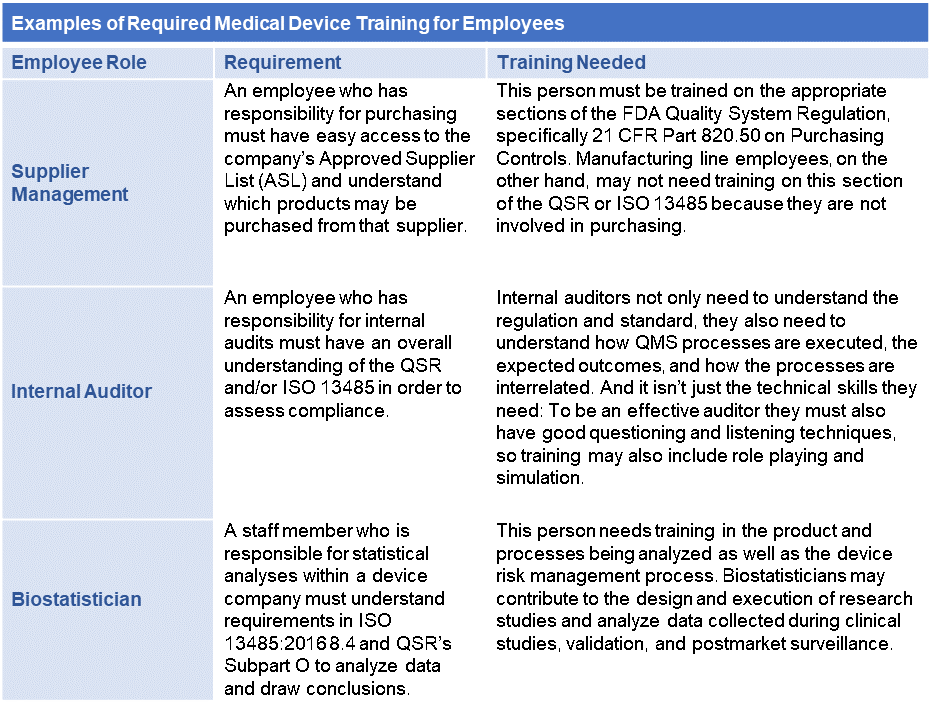
EU MDR: EU Medical Device Regulation - Chapter 1: Scope and Definitions Training Certification Course

Asia Training Center for Pharmaceuticals and Medical Devices Regulatory Affairs | Pharmaceuticals and Medical Devices Agency