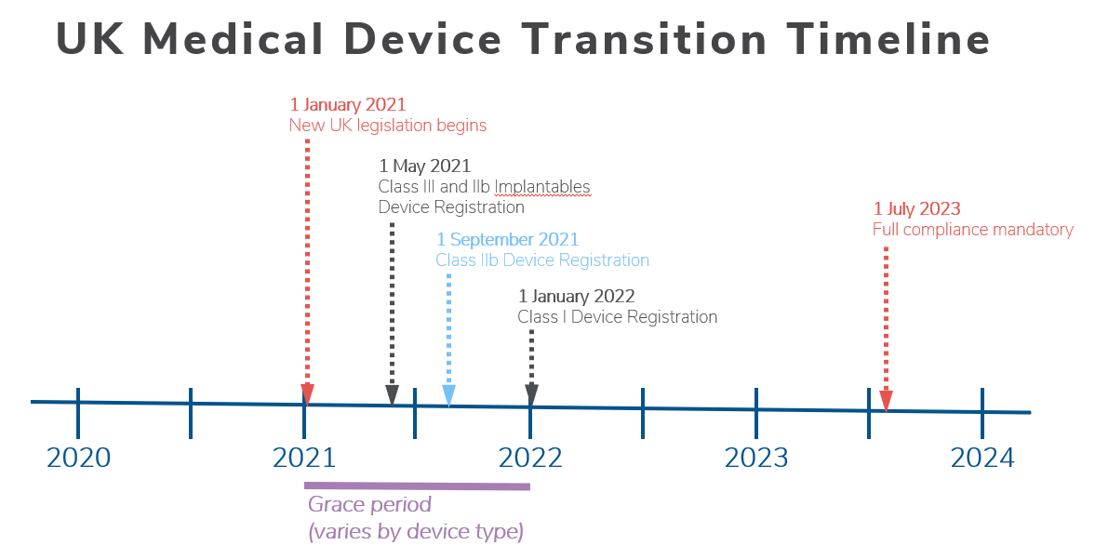
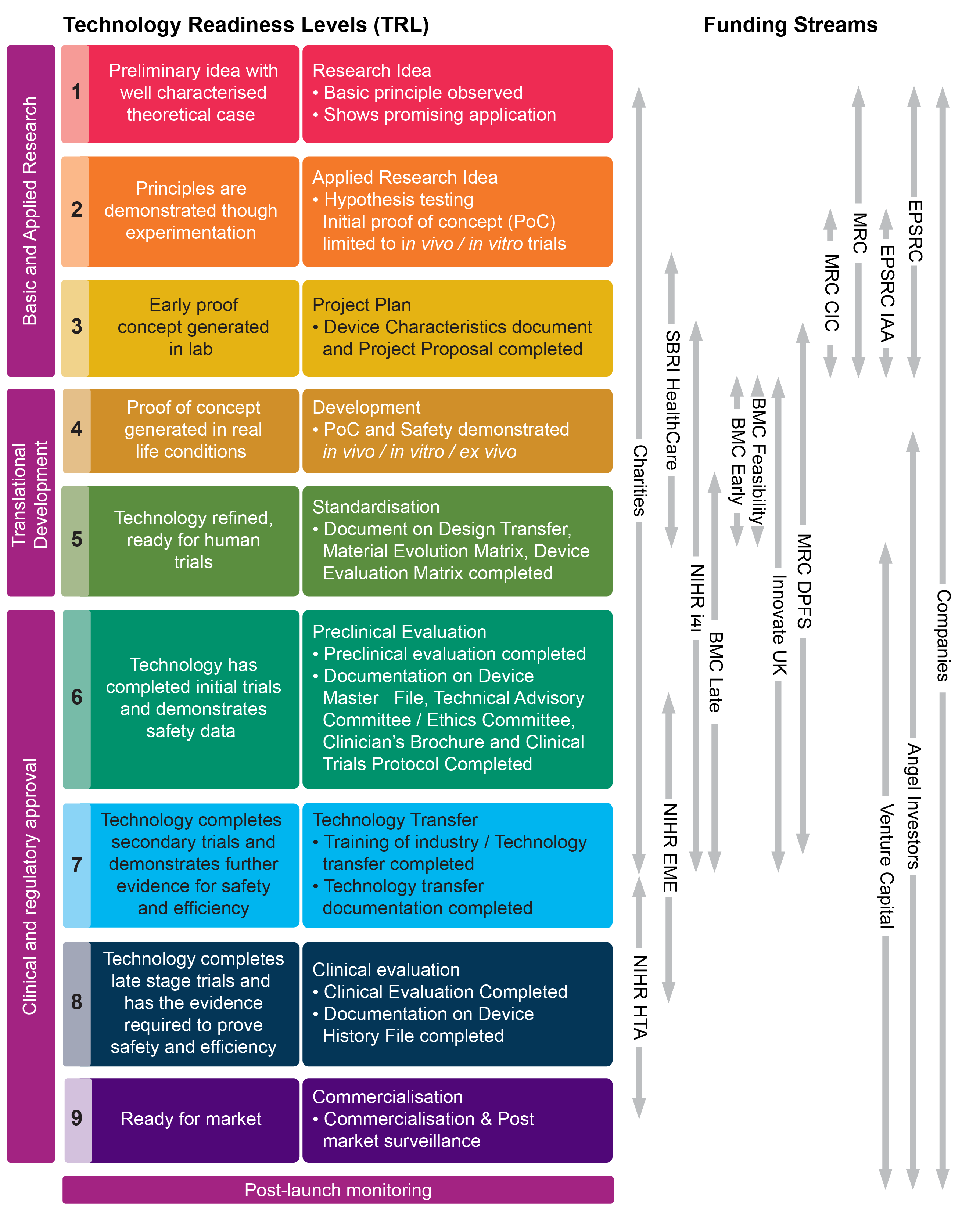
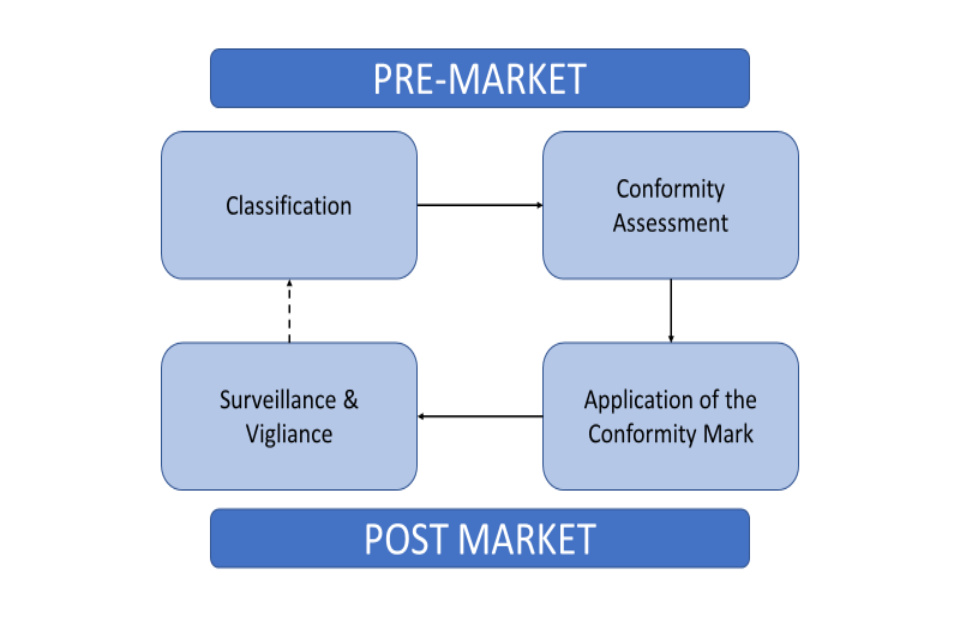
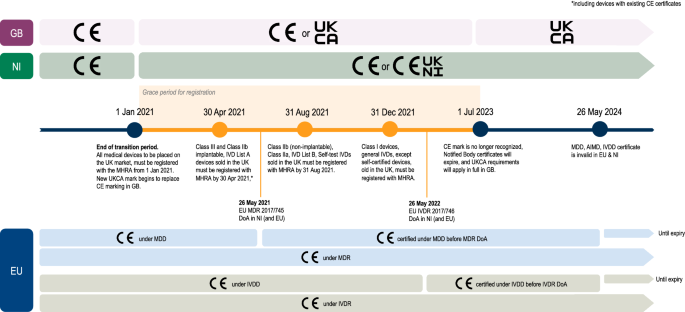
Medical Device “Certificates of Compliance” / “Attestation of Conformity” have no legal standing under UK Medical Device Regulations 2002 - GOV.UK

AXREM summary of the MHRA response to the consultation on the future regulation of medical devices in the UK - AXREM


-png.png)