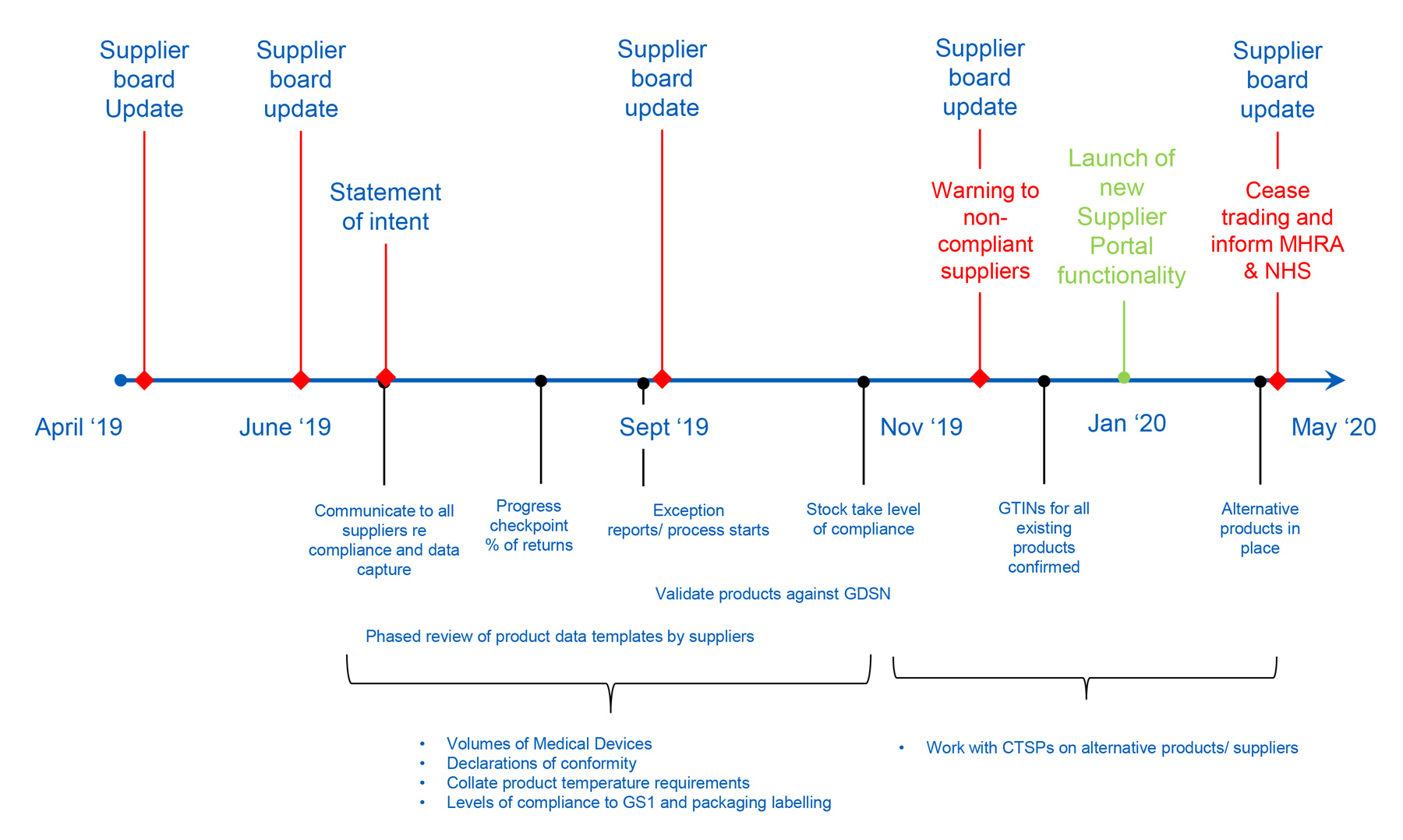
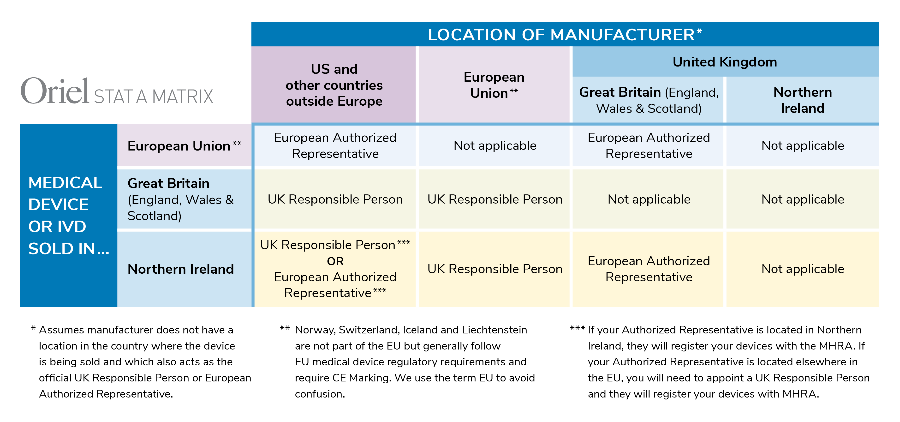
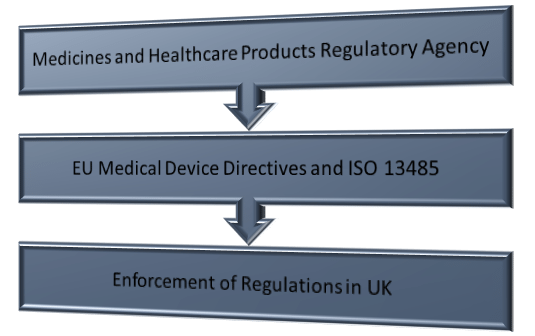
Flow chart to support navigation of regulatory requirements by academic... | Download Scientific Diagram

MHRA revamps UK clinical trial regulation with the promise of faster timelines - Pharmaceutical Technology

MHRA Publishes Post-transition Period Regulations for Clinical Trials | Aariya Regulatory Services Pvt Ltd

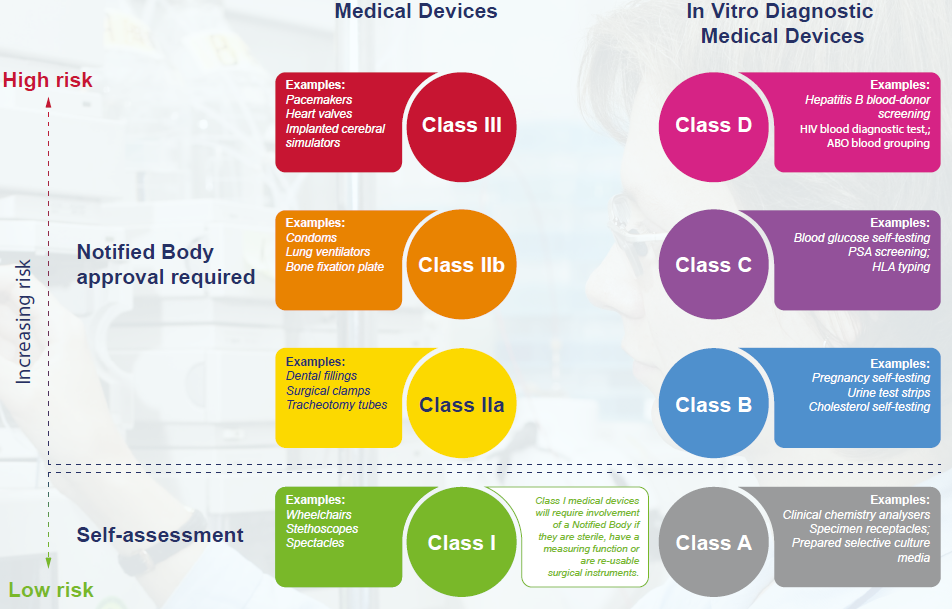
MHRA Medical Devices Consultation | UCL Institute of Healthcare Engineering - UCL – University College London
MHRA to streamline clinical trial approvals in biggest overhaul of trial regulation in 20 years - GOV.UK

AXREM summary of the MHRA response to the consultation on the future regulation of medical devices in the UK - AXREM