Surface tension as a function of the molal concentration of AN for the... | Download Scientific Diagram

The molar concentration of chloride ions in the resulting solution of 300mL of 3.0M NaCl and 200 mL of 4.0MBaCl2 will be :

Solutions Concentrations of Solutions. Solutions Objectives Given the mass of solute and volume of solvent, calculate the concentration of solution. - ppt download

Al(SO)(4))(3) solution of 1 molal concentration is present in 1 litre solution of density 2.684 g//cc. How many moles BaSO(4) would be precipated on adding excess BaCl(2) in it?

Preview Objectives Concentration Molarity Molality Chapter 12 Section 3 Concentration of Solutions. - ppt download

Henry's constant for argon is 40k bar in water determine molal concentration of argon in water when it is stored above water at 10 bar pressure

Viscosity versus molal concentration of the salt at temperature and... | Download Scientific Diagram
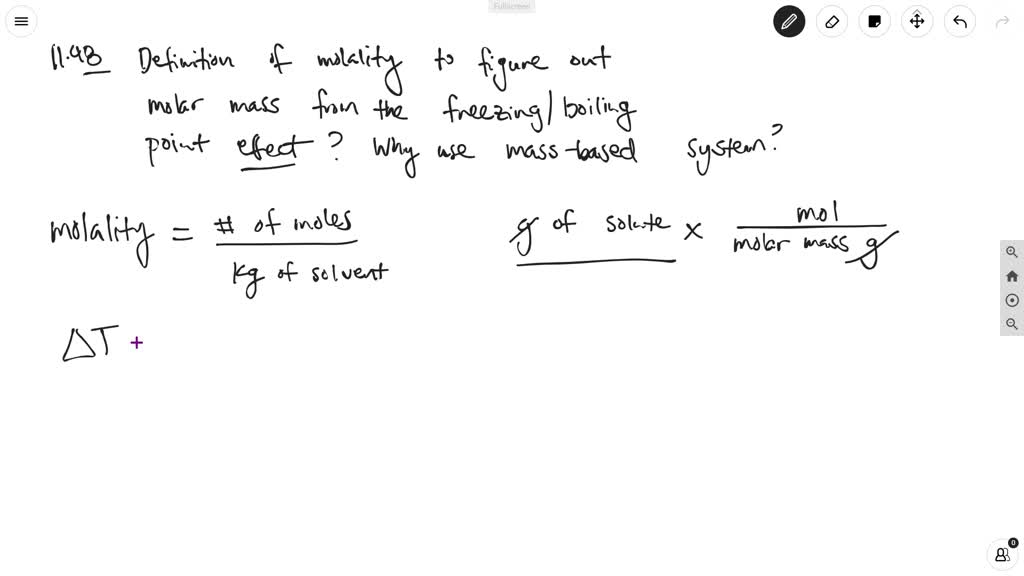
SOLVED: Molality, also called molal concentration, is a measure of the concentration of a solute, in a solution in terms of amount of substance in a specified amount of mass of the

Al(SO)(4))(3) solution of 1 molal concentration is present in 1 litre solution of density 2.684 g//cc. How many moles BaSO(4) would be precipated on adding excess BaCl(2) in it?
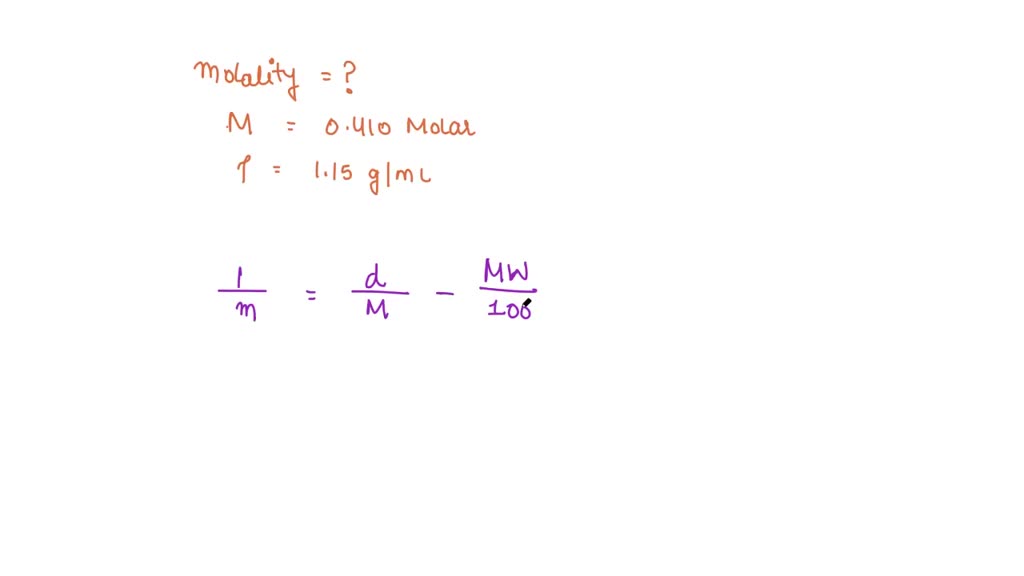
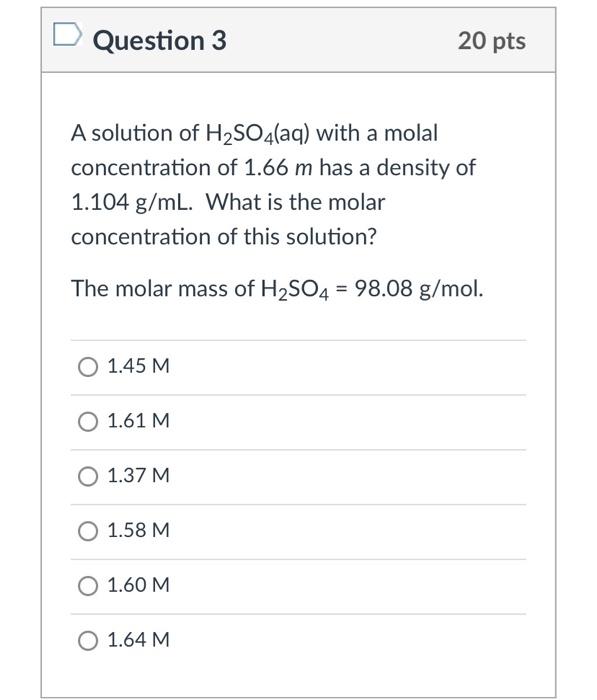
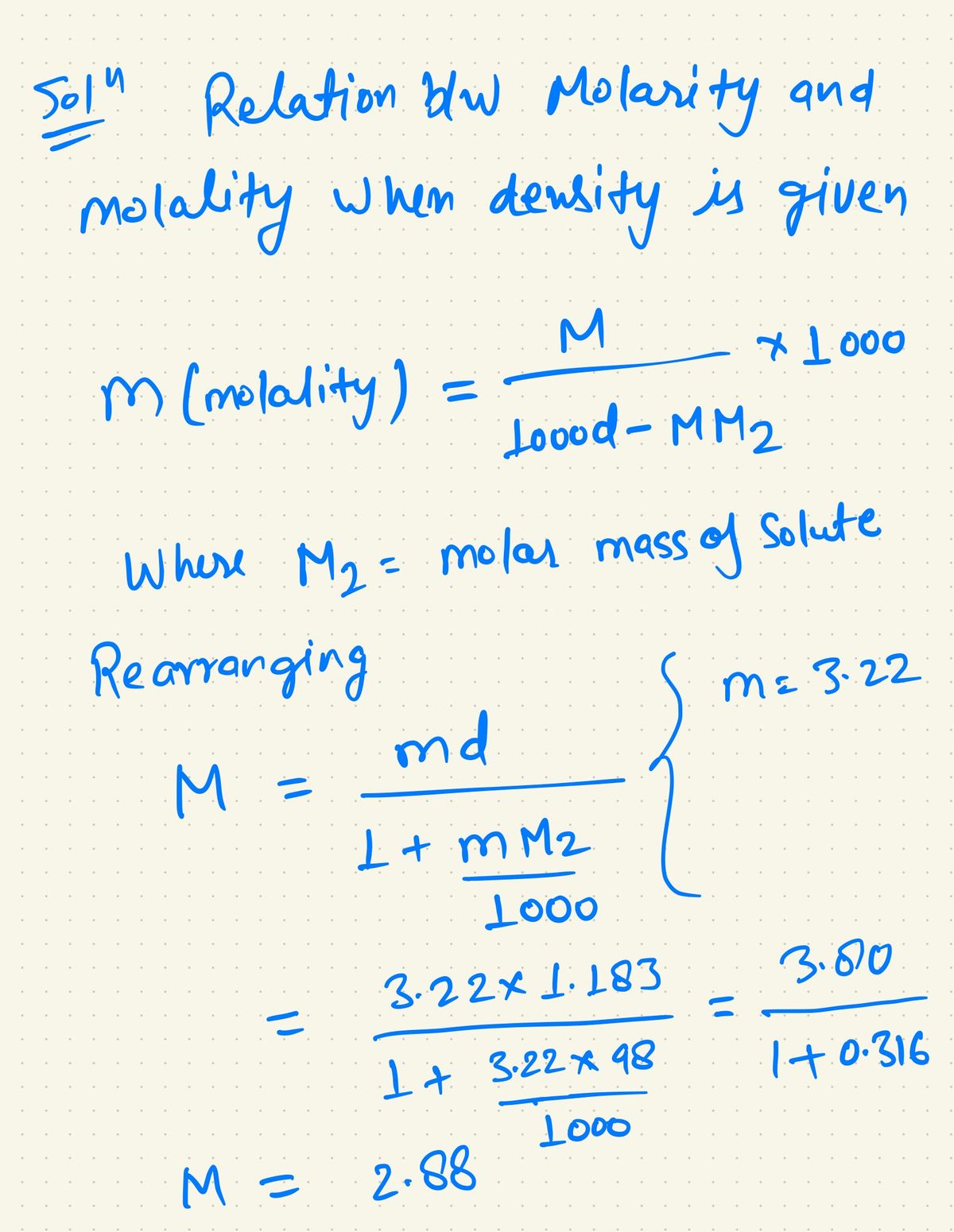
SOLVED: "1 What (s the molal concentration of 5.3 M HBr in an aqucous solution whose Elcm'? density Is 1.5 2 Determine the molarity of J 13.2 m Na,HPO aqueous solution whose
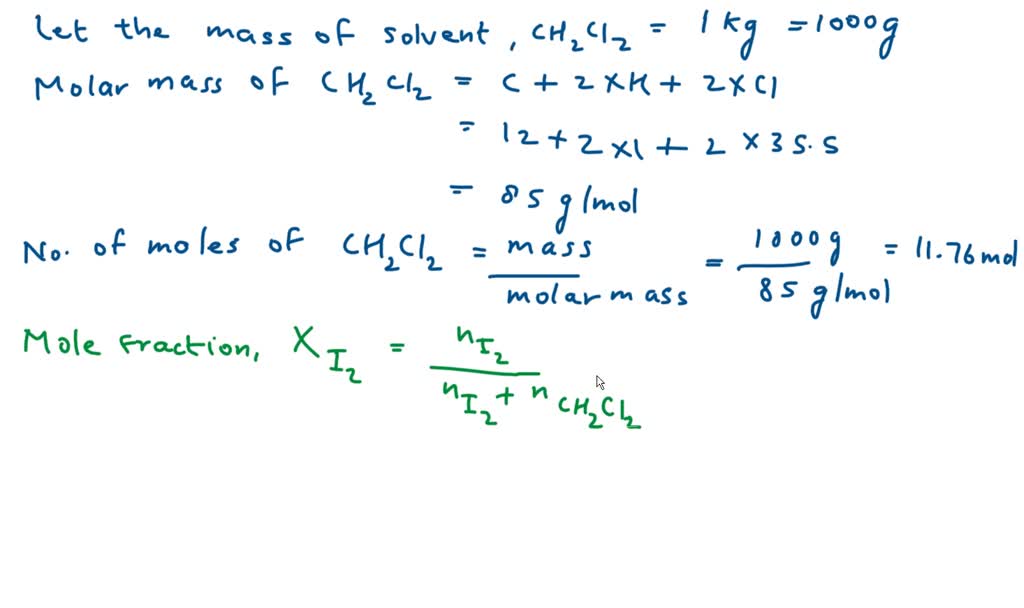
SOLVED: The mole fraction of iodine, I2, dissolved in dichloromethane, CH2Cl2, is 0.115. What is the molal concentration, m, of iodine in this solution?
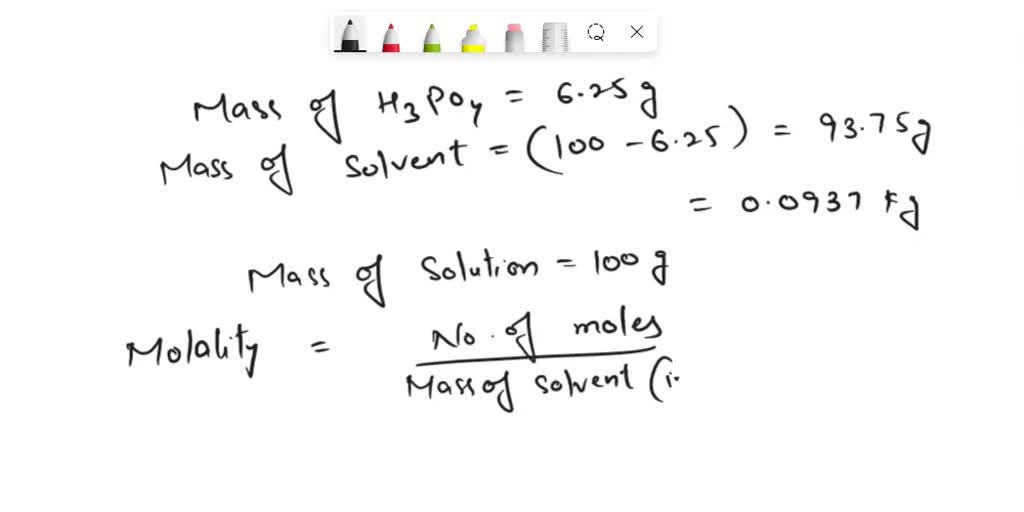
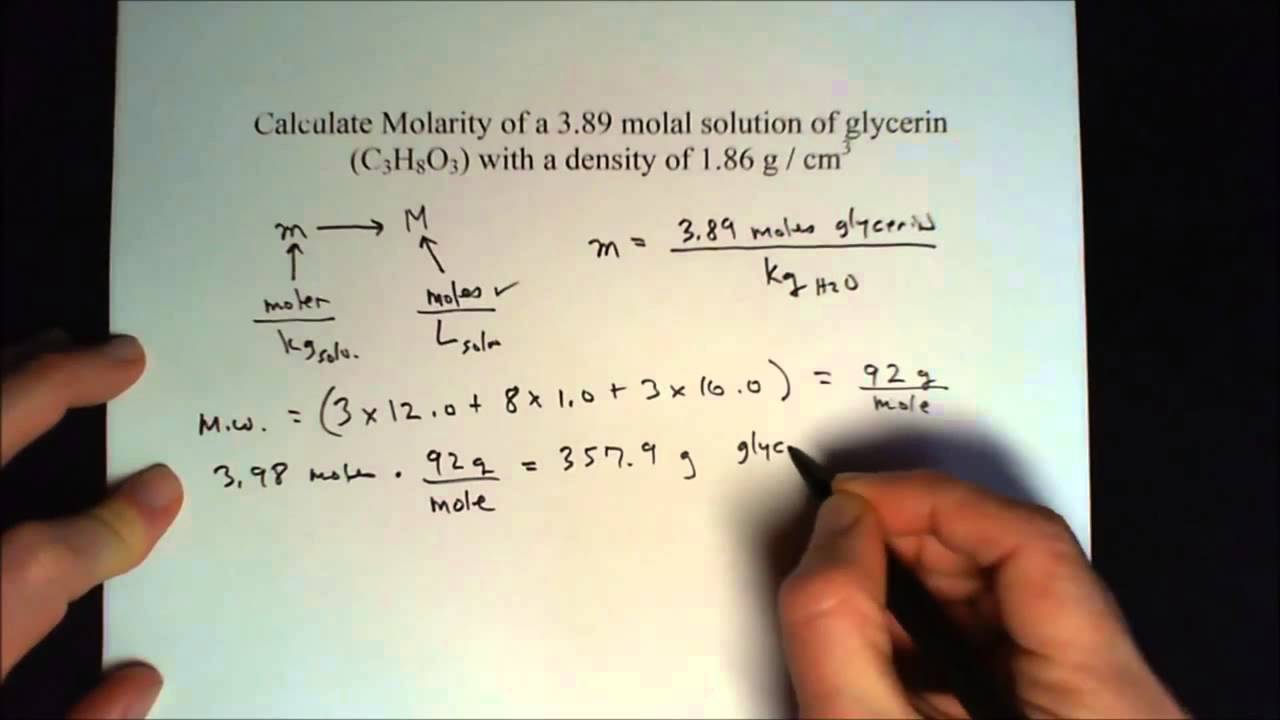
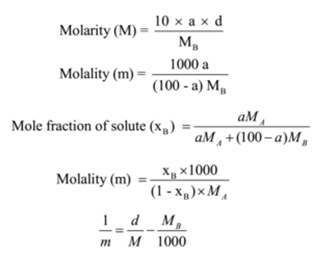
SOLVED: Calculate the molal concentration, molar concentration, and mole fraction of 6.25% solution of H3PO4 (density= 1.06 g/mL).