COMMISSION IMPLEMENTING REGULATION (EU) 2017/ 2185 - of 23 November 2017 - on the list of codes and correspond
Codes assignment referred to in the implementing Regulation 2017/2185 in the context of a request for certification according to the Regulation (EU) 2017/745 - GMED Medical Device Certification

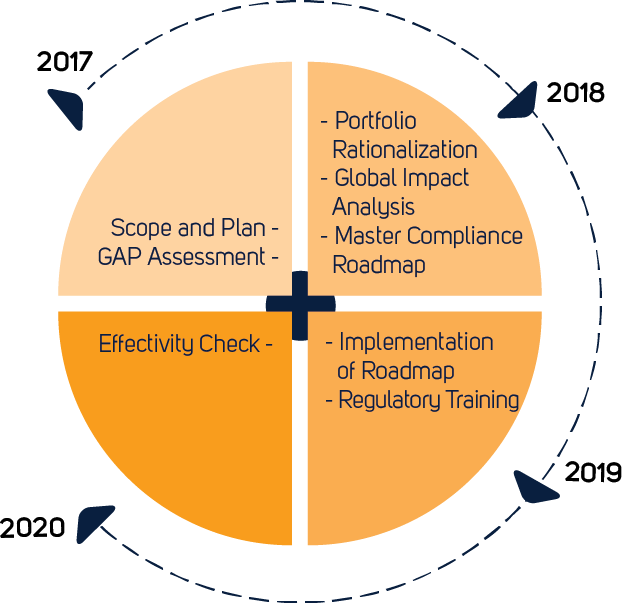
Europe - Joint Implementation/preparedness plan on the new Medical Devices Regulation 2017/745 (MDR) on March 11th, 2020 - RIS.WORLD

IVD Directive to IVD Regulation (EU 2017/746) Transition – 8 Months Remaining - Voisin Consulting Life Sciences

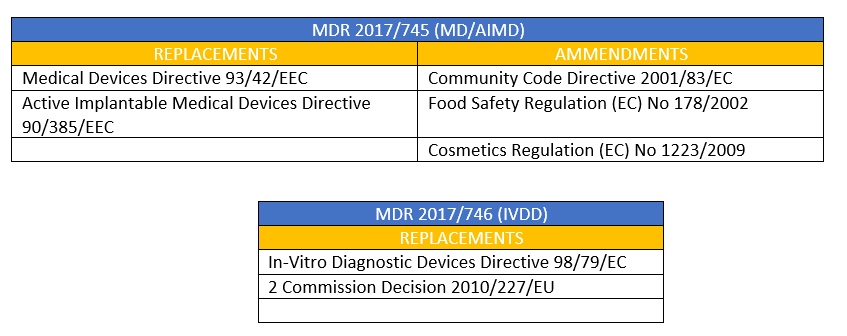
MDR 2017/745 - Article 120: Provisions on the marketing of devices and validity of EC certificates - Ente Certificazione Macchine

The European Parliament has adopted the proposed amendment to the MDR to extend the transitional period for legacy Medical Devices

COAL MINES REGULATION 2017 WITH IMPORTANT CMR 2017 OBJECTIVES ( UPDATED EDITION 2021) : ch rs sharma: Amazon.in: Books

Easy Access Rules for Air Traffic Management/Air Navigation Services ( Regulation (EU) 2017/373) - Revision from February 2023 - Available in pdf, online & XML format | EASA

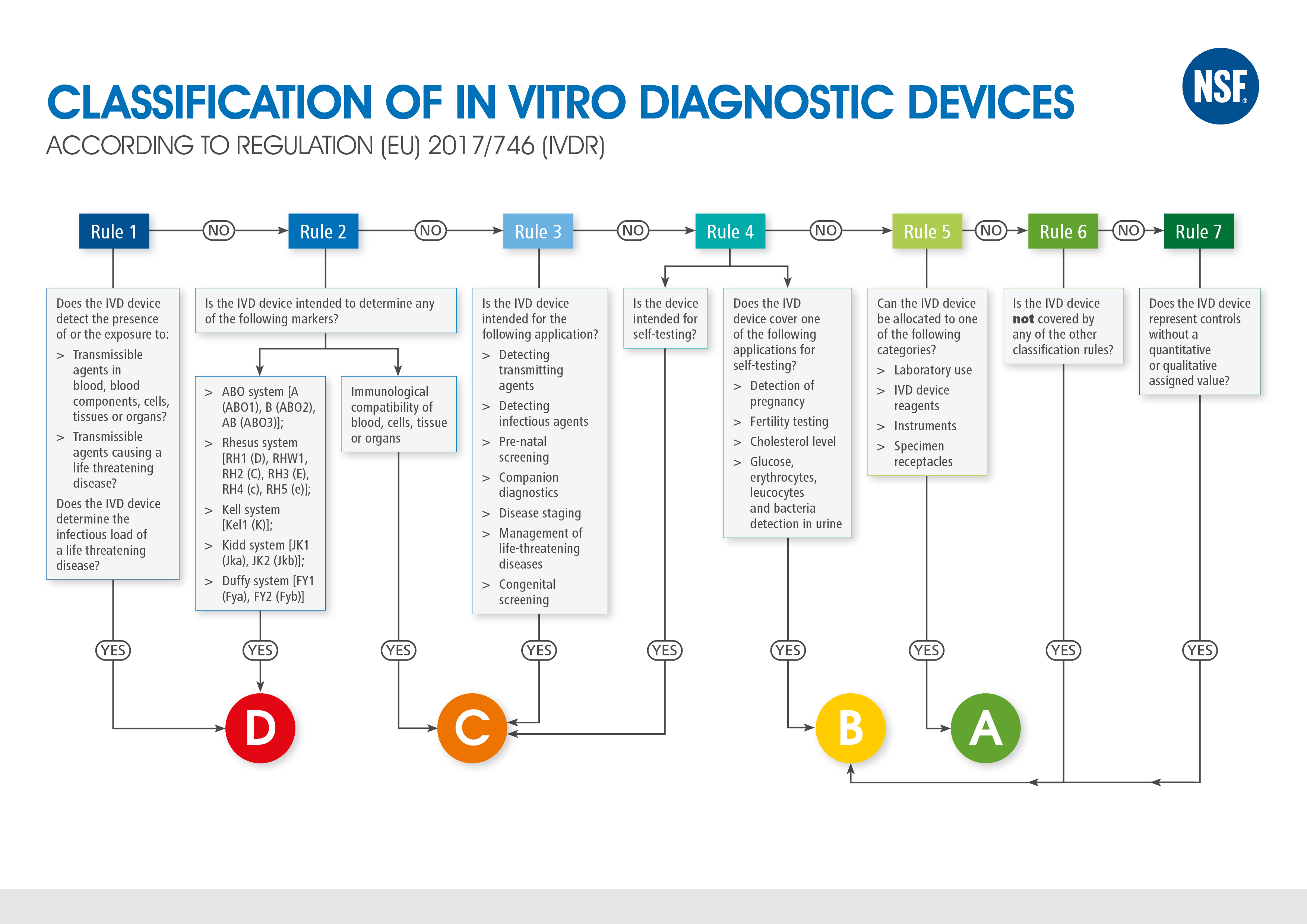
In vitro diagnostic software: Novelties introduced by Regulation (EU) 2017/746 - GMED Medical Device Certification