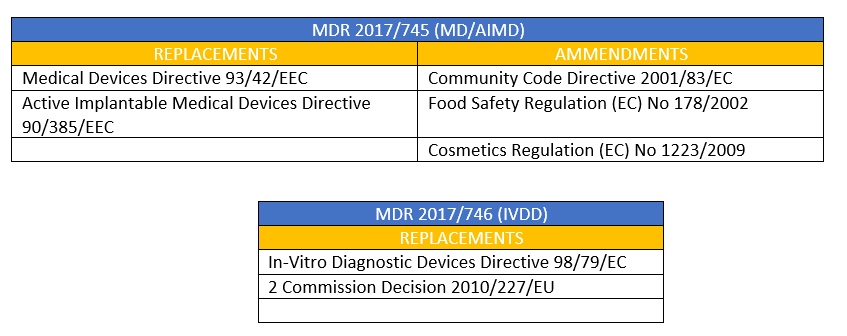
Corrigendum to Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amen

Guerbet - Statement regarding compliance to the European Medical Device Regulation (Regulation (EU) 2017/745, MDR) May 26th, 2021

Europe - Data generated from 'Off-Label' Use of a device under the EU Medical Device Regulation 2017/745 - RIS.WORLD

Medical Devices Clinical Evaluation - Summary of Safety and Clinical Performance (SSCP) - Regulation (EU) 2017/745 - GMED Medical Device Certification

Webinar - Materiovigilance: Everything you need to know about the new European Regulation on Medical Devices (EU Regulation 2017/745) - Universal Medica
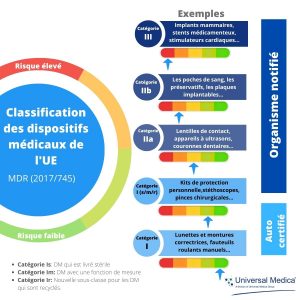
Points clés du nouveau règlement européen sur les dispositifs médicaux (UE) 2017/745. - Universal Medica




![EU MDR 2017/745 Transition timeline [Medical Device Regulation] EU MDR 2017/745 Transition timeline [Medical Device Regulation]](https://easymedicaldevice.com/wp-content/uploads/2018/10/MDR-Transition-timelineV1Slice_01.png)