Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU)

Nouveaux règlements européens pour les dispositifs médicaux - ANSM : Agence nationale de sécurité du médicament et des produits de santé

Italy: Bills to implement Regulation (EU) 2017/745 and Regulation (EU) 2017/ 746 on medical devices and in vitro diagnostic medical devices - Global Compliance News
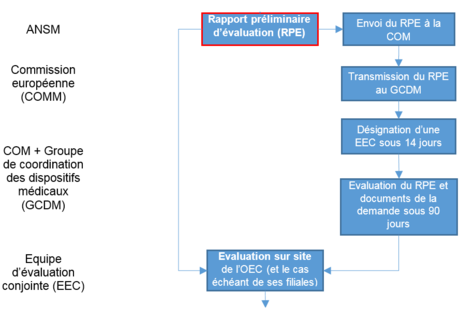
Évaluation, désignation et notification des organismes d'évaluation de la conformité en France - ANSM

In vitro diagnostic software: Novelties introduced by Regulation (EU) 2017/ 746 - GMED Medical Device Certification

Europe - Manual on borderline and classification for medical devices under Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (Revised) - RIS.WORLD
Questions & Answers on Implementation of the Medical Devices and In Vitro Diagnostic Medical Devices Regulations ((EU) 2017/
IDS049 - Élaboration de la Documentation Technique selon le Règlement 2017/ 746 - Mise à jour et amélioration du Système de Management de la Qualité selon ISO 13485 : 2016 - Bibliothèque des travaux Master
Transition to EU IVD Regulation (EU) 2017/746 and considerations for non-EU regulatory authorities on managing the impact to pro

IVD Directive to IVD Regulation (EU 2017/746) Transition – 8 Months Remaining - Voisin Consulting Life Sciences