September 2021 The rules governing medicinal products in the European Union VOLUME 10 - Guidance documents applying to clinical
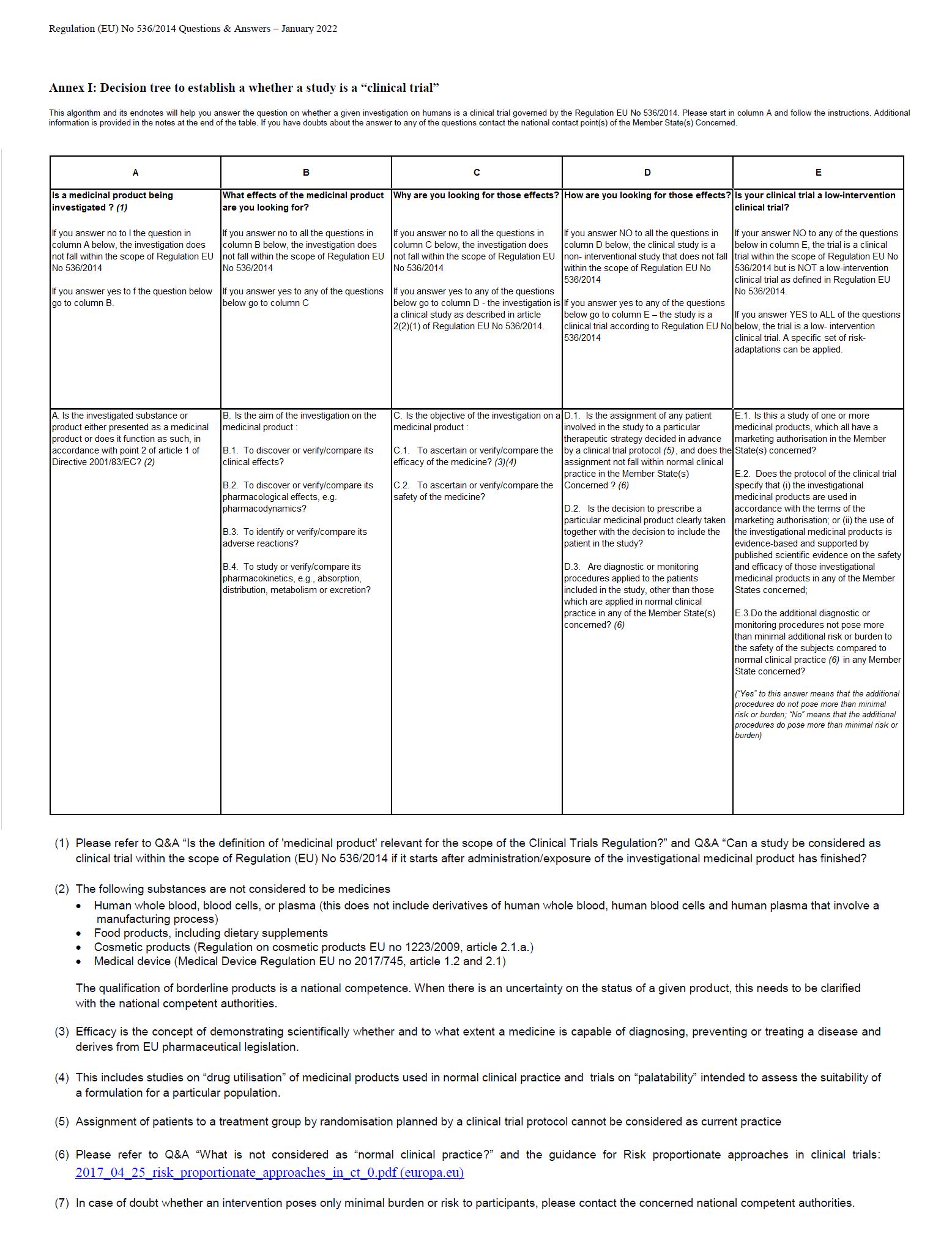
Decision tree to establish a whether a study is a clinical trial – Uit Annex I van de Q&A Regulation (EU) No 536/2014 (versie april 2022) – Vincent Bontrop

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging