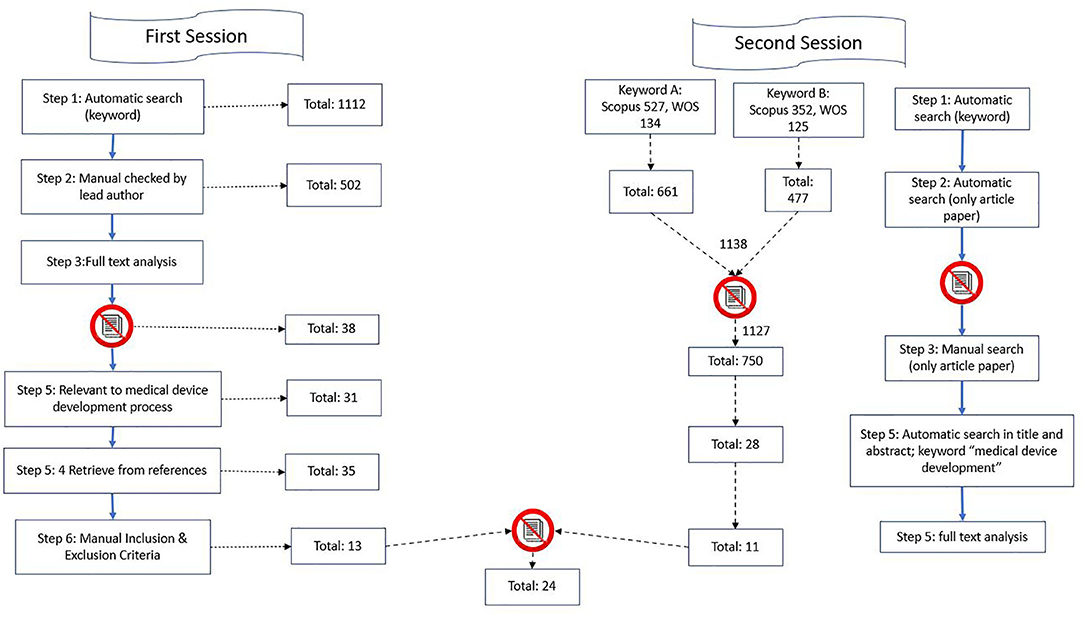
Frontiers | Medical Device Development Process, and Associated Risks and Legislative Aspects-Systematic Review

South Korea - South Korea updates regulations on medical device codes and classification - RIS.WORLD

Case Study 24 : Registration of a Software as medical device in China, Columbia and South Korea for European AI based Company - Global Regulatory Partners, Inc.
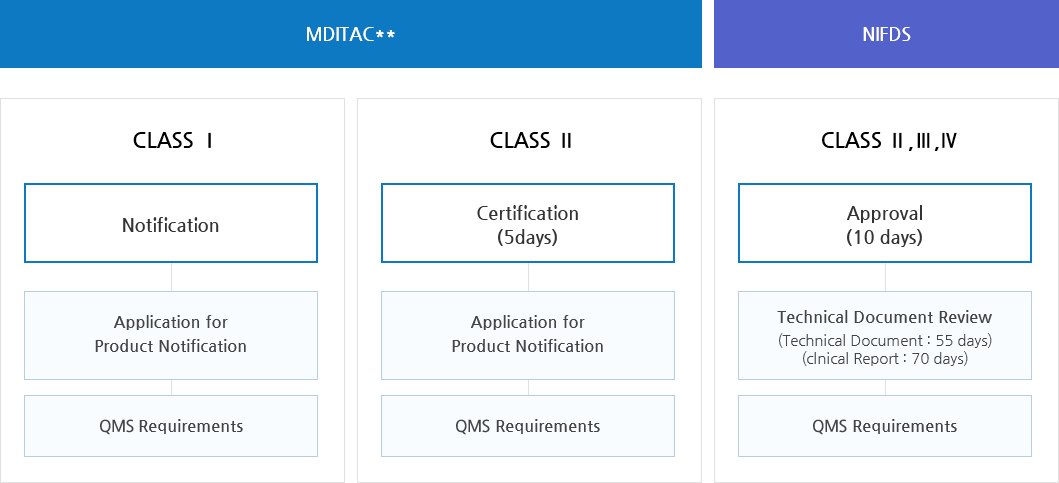
Ministry of Food and Drug Safety>Our Works>Medical Devices>Approval Process | Ministry of Food and Drug Safety