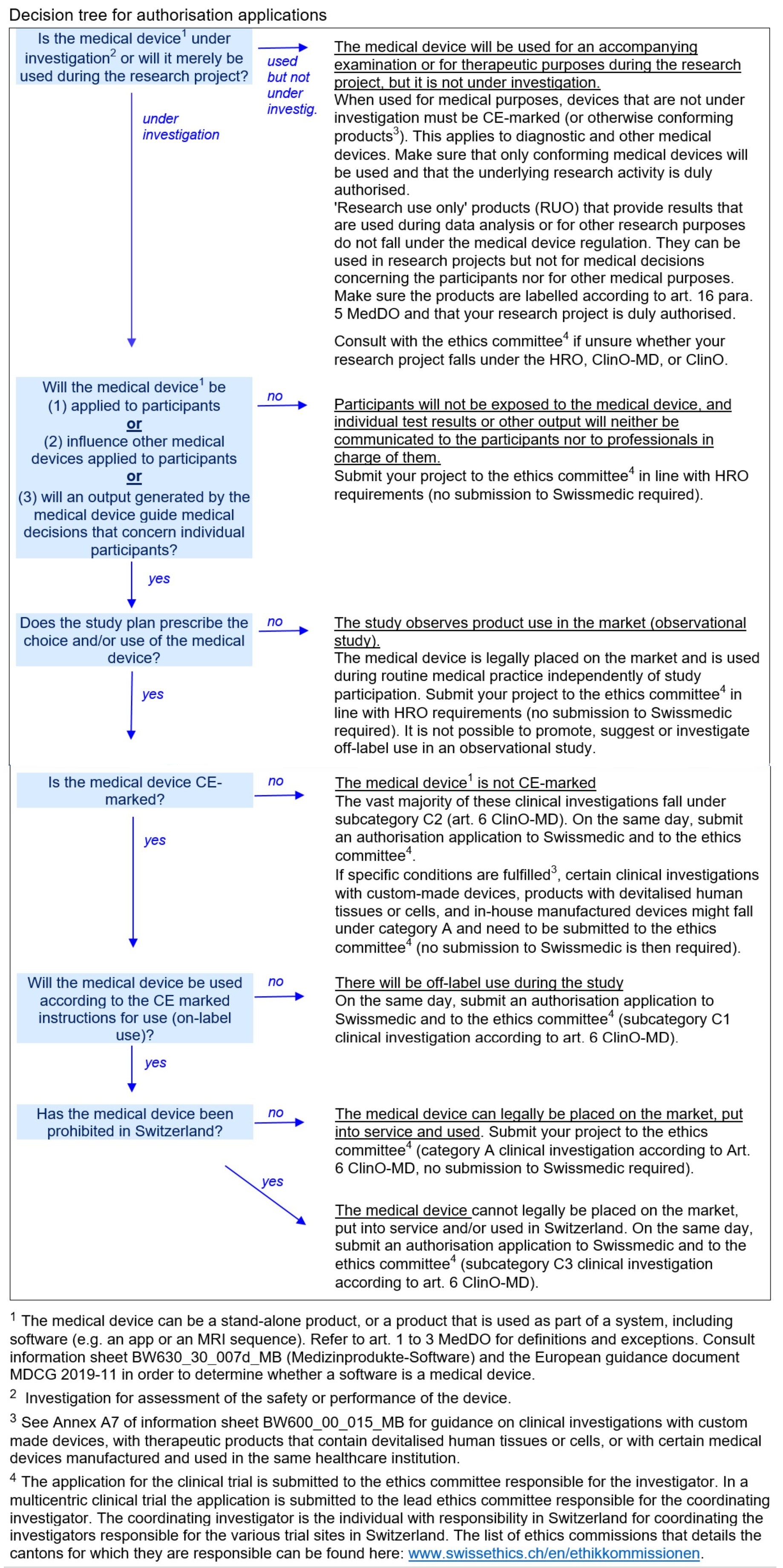
Meditrial on Twitter: "#Switzerland: On May 26, 2022, the new Regulation on #InVitroDiagnostic #MedicalDevices (#IvDV) entered into force. At the same time, the revised Ordinance on #ClinicalTrials of Medical Devices (#KlinVMep) came
EUROPEAN COMMISSION Brussels May 2022 Until now, Switzerland has been participating in the European Union internal market for in





/tuv-rheinland-ivdr-visual-1-en.png)